The Nobel Prize in Chemistry 1918.
"for the synthesis of ammonia from its elements"
 Fritz Haber (1868 - 1934) was awarded the 1918 Nobel Prize in Chemistry for working out a method to synthesize ammonia (NH3) from nitrogen (N2) and hydrogen (H2). This process, now known as the Haber-Bosch process, is an essential step in the production of artificial fertilizer. At the time, it was not known how long the natural sources of nitrogen such as Chile saltpetre (sodium nitrate) would last. Harber worked at the Kaiser-Wilhelm-Institut (now Fritz-Haber-Institut) für physikalische Chemie und Electrochemie in Berlin-Dahlem, Germany
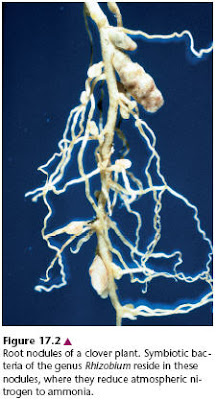
Fritz Haber (1868 - 1934) was awarded the 1918 Nobel Prize in Chemistry for working out a method to synthesize ammonia (NH3) from nitrogen (N2) and hydrogen (H2). This process, now known as the Haber-Bosch process, is an essential step in the production of artificial fertilizer. At the time, it was not known how long the natural sources of nitrogen such as Chile saltpetre (sodium nitrate) would last. Harber worked at the Kaiser-Wilhelm-Institut (now Fritz-Haber-Institut) für physikalische Chemie und Electrochemie in Berlin-Dahlem, GermanyMany species of bacteria can fix nitrogen into ammonia in a reaction catalyzed by the enzyme nitrogenase [The Nitrogen Cycle]. The Haber-Bosch process involves heating nitrogen and hydrogen to a temperature of 450°C under 200 atm of pressure in the presence of an iron catalyst. Bacteria do it at 20°C or less at atmospheric pressure.
The 1918 prize was announced in November 1919 and the prize wasn't awarded until June 1920. Part of the delay was due to the fact that Fritz Haber was head of the German Chemical Warfare Service during the war. He was responsible for initiating mustard gas attacks on the allied lines.
The presentation speech was delivered by Doctor Å.G. Ekstrand, President of the Royal Swedish Academy of Sciences. No member of the Swedish Royal family was present at the ceremony because of the death of crown-princess Margaret.THEME:
Nobel Laureates
The Royal Swedish Academy of Sciences has decided to confer the Nobel Prize in Chemistry for 1918 upon the Director of the Kaiser Wilhelm Institute at Dahlem near Berlin, Geheimrat Professor Dr. Fritz Haber, for his method of synthesizing ammonia from its elements, nitrogen and hydrogen.
In accordance with Nature's plan of economy, soil fertility under normal circumstances is maintained at an even level if the waste products from the crop are returned to the soil; if, however, substantially increased productivity is required from the soil, then additional fertilizer must be used. Since meanwhile a large proportion of the annual harvest is consumed by the yearly increasing population of towns, and since the towns' waste products are returned to land under cultivation only to a very incomplete extent, the inevitable consequence is that the soil becomes exhausted and the harvest yield diminishes. This has, in turn, led to the manufacture of artificial fertilizers which has also increased year by year in importance to such an extent that, at least in Europe, hardly a country exists which can do entirely without them.Among these substances nitrogenous compounds occupy an important position, since usually the soil does not possess a large store of these to be released to suit the plants' needs by weathering as in the case of phosphoric acid and potash; added to which there is the fact that part of the effective nitrogen turns into inactive atmospheric nitrogen during the cyclic process. Admittedly a part of this loss is compensated by rainfall and through the activity of bacteria, but so far experience has shown that intensive cultivation cannot be maintained without artificial nitrogenous fertilizers. This applies, above all, to one of today's most important crops, sugar-beet.
For many years only two artificial nitrogenous compounds existed, namely potassium nitrate and ammonium chloride. The older methods by which these were made, however, ceased to play a part, at least in Europe and America, when Chile saltpetre (sodium nitrate) came into the picture and use was made of the by-products from dry distillation of mineral coal for this purpose.The consumption of Chile saltpetre, calculated in terms of nitrogen, amounts to about 500,000 or more tons per annum. Under normal circumstances the vast majority of this saltpetre is used for fertilizer purposes. The burning question, therefore, has long been: how long will the saltpetre deposits in Chile last? The Chilean authorities give very widely varying estimates, and experts in Europe are of the opinion that at current production rates the deposits will be exhausted within the foreseeable future.
Be that as it may. The protracted World War has sufficiently demonstrated to every country the need of organizing, wherever possible, production of essential commodities within its own borders in sufficient quantities to meet its own needs.
Now, since saltpetre is among the most important of these substances, particularly in those countries which possess neither large mineral coal deposits nor cheap hydro-electric power, the artificial production of ammonia and nitric acid has reached an unprecedented degree of importance.
A substance on the borderline between natural and artificial products is the ammonia obtained by dry distillation of bituminous and brown coal. This ammonia comes from the nitrogen content of these minerals, amounting to approximately 1.3 % by weight, of which however the largest portion (around 85%) remains behind in the coke or is liberated as nitrogen during distillation.
During the first ten years of this century several methods were published, based on binding the nitrogen from the air, but few of these survived the trial stage. The first of these was Frank-Caro's cyanamide method. Indeed it appears that calcium cyanamide did not come fully up to expectations as a fertilizer, but since its nitrogen content can be converted to ammonia relatively easily, this has not so far proved to be an obstacle to the application of the method to an ever-increasing extent.
Using the main principles of thermodynamics every quantitative condition with regard to the combustion of atmospheric nitrogen to produce nitric oxide can be calculated. Birkeland and Eyde were, of course, the first to apply this technically with successful results.
Until 1904 nobody had been able to bring about a direct combination of nitrogen and hydrogen to form ammonia without the help of dark electrical discharge, although the experiments of Berthelot and Thomson proved that the combination occurred exothermically. With the experience we now have we can easily see that this negative result was due to the slowness of the reaction at low temperatures, and unfavourable equilibrium conditions at high temperatures. Admittedly, in 1884 Ramsay and Young had conducted some experiments on this, using iron fillings as a catalyst, but these yielded only uncertain results.
In 1904 Haber and van Oordt began a methodical study of this relevant field, based on modern physico-chemical methods, after a single previous experiment had given Haber a hope of finding a technical solution to the problem. They worked at a temperature of about 1,000° C and normal pressure, using iron as a catalyst. From these experiments it emerged that from red heat onwards, and also at higher pressures, only traces of ammonia could be formed.
During this work it was also shown experimentally for the first time that a real state of equilibrium existed in the system
N2+ 3H2 → NH3, which is in fact the real basis for the synthesis of ammonia.
In the "Zeitschrift für Elektrochemie" of 1913 can be found the treatment of this question, by Haber and Le Rossignol which has the most important practical meaning: "Über die technische Darstellung von Ammoniak aus Elementen" (On the technical production of ammonia from the elements). This treatise provided the groundwork for the development of the method on a factory scale at the "Badische Anilin- und Sodafabrik" in Ludwigshafen, the main development occurring under the guidance of Dr. C. Bosch.
Earlier experiments had shown the pointlessness of exceeding dark red heat, i.e. about 600° C. On the other hand, the reaction formula showed that combination occurs with a contraction of from 4 to 2 volumes.
From the law of equilibrium it follows that the higher the pressure is the more the equilibrium must shift to the ammonia side. This provided the basic principles. A temperature of about 500° C had to be used at the highest possible pressure, which in practice meant at about 150-200 atmospheres. It could also be assumed that this high pressure speeded up the reaction. But work with a flow of gas in a circulation system at such high pressure and at a temperature approaching red heat posed very severe difficulties and up to then had never been tried. It was, however, completely successful. The treatise in question contains detailed drawings of the equipment used with which, using iron as a catalyst, about 250 grams of ammonia were produced per hour and per litre of contact volume; with uranium or osmium as a catalyst considerably more was produced.
The heating is done electrically. Since however the heat escaping from the equipment is largely regenerated in the entrant gases the required temperature can largely be maintained by the regenerated heat and by the heat liberated during the formation of ammonia. A very important point in Haber's observations is that the gases can be given a greater flow rate during the reaction which of course substantially increases the amount of ammonia produced per unit of time.
Haber found the best catalyst to be osmium, followed by uranium or uranium carbide. According to tests conducted mostly at the factories of the "Badische", the activity of the catalyst may be increased by oxides or certain salts of alkalis and alkaline earth metals, just as it may be decreased by catalytic poisons. Gradually more active catalysts have been discovered, and by this means it has been found possible to reduce substantially the pressure in the chamber.
In 1910 construction work was begun on the first large factory near Oppau in the neighbourhood of Frankfurt am Main, with an estimated annual output of 30,000 tons of ammonia.
The basic materials, nitrogen and hydrogen, are produced by standard methods.
Power consumption in the ammonia process is very low, amounting to no more than 0.5 kilowatt-hours per kilogram of ammonia. Per kilowattyear, therefore, no less than 10,000 kilograms of nitrogen are bound.
Since the position of the equilibrium of the reaction depends, among other things, upon the heat of formation of ammonia and its specific heat, Haber in a series of seven articles in the "Zeitschrift für Elektrochemie" of 1914-1915, has extensively described experiments carried out to confirm these figures with the greatest possible accuracy.
As, according to Ostwald's modified method, ammonia can be converted into nitric acid and the latter into calcium nitrate, the ratio between the overall costs of producing calcium nitrate is, according to the available calculations, approximately as follows:
Norwegian Hydro: 100
Haber: 103
Frank-Caro: 117
in other words, they are the same for the first two methods but approximately 15% higher for the last.
Since, however, of the three existing nitrogen methods, Haber's is the only one capable of operating independently of any available source of cheap hydroelectric power it can in future be applied in all countries; since furthermore it can be utilized on any convenient scale and because it can produce ammonia very much more cheaply and nitrate equally as cheaply as any other method, as explained above, it is of universal significance for the improvement of human nutrition and so of the greatest benefit to mankind.
German Haber factories, especially the recently built Leuna Works near Merseburg, are also in full production, providing the vast majority of all nitrogenous fertilizers obtainable in Germany. Moreover, the method has already been extensively applied in the United States of America.