Some textbooks do not show the uric acid degradation pathway since it doesn't occur in humans and those textbooks aren't interested in an evolutionary approach to biochemistry (e.g. Berg, Tymoczko, and Stryer). The other majors textbooks (Voet & Voet, Garrett & Grisham, Nelson & Cox [Lehinger]) all show uric acid converted directly to allantoin via urate oxidase. This reaction was shown to be incorrect about 15 years ago. The actual pathway from uric acid to allantoin involves two intermediates; 5-hydroxyisourate and OHCU.
Image Credit: Moran, L.A., Horton, H.R., Scrimgeour, K.G., and Perry, M.D. (2012) Principles of Biochemistry 5th ed., Pearson Education Inc. page 568 [Pearson: Principles of Biochemistry 5/E] © 2012 Pearson Education Inc.The winner, for the second week in a row, is Jean-Marc Neuhaus. [Monday's Molecule #220]. Jean-Marc lives in Switzerland so I've made arrangements to fly over there to visit him and treat him to two fondues at the Pinte de Pierre-à-Bot in Neuchatel.
 Jean-Marc was kind enough to send me a menu [PDF]. There are about 30 different fondues to choose from. If you would like to join us you can leave a comment on last week's post.
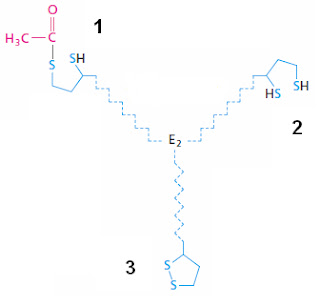
Jean-Marc was kind enough to send me a menu [PDF]. There are about 30 different fondues to choose from. If you would like to join us you can leave a comment on last week's post. This week's molecule is related to a discussion we are having on the How Do the IDiots Explain the Origin of Life? post. Can you identify this molecule? You have to be very specific.
Email your answer to me at: Monday's Molecule #221. I'll hold off posting your answers for at least 24 hours. The first one with the correct answer wins. I will only post the names of people with mostly correct answers to avoid embarrassment. The winner will be treated to a free lunch.
There could be two winners. If the first correct answer isn't from an undergraduate student then I'll select a second winner from those undergraduates who post the correct answer. You will need to identify yourself as an undergraduate in order to win. (Put "undergraduate" at the bottom of your email message.)