 This is part 10 of my review of The Myth of Junk DNA. For a list of other postings on this topic see the links below. For other postings on junk DNA check out the links in Genomes & Junk DNA in the "theme box" below or in the sidebar under "Themes."
This is part 10 of my review of The Myth of Junk DNA. For a list of other postings on this topic see the links below. For other postings on junk DNA check out the links in Genomes & Junk DNA in the "theme box" below or in the sidebar under "Themes." More Recent Comments
Monday, August 22, 2011
Junk & Jonathan: Part 10—Chapter 7
 This is part 10 of my review of The Myth of Junk DNA. For a list of other postings on this topic see the links below. For other postings on junk DNA check out the links in Genomes & Junk DNA in the "theme box" below or in the sidebar under "Themes."
This is part 10 of my review of The Myth of Junk DNA. For a list of other postings on this topic see the links below. For other postings on junk DNA check out the links in Genomes & Junk DNA in the "theme box" below or in the sidebar under "Themes." What Ever Happened to Freya?
 Freya was one of the important Norse gods.
Freya was one of the important Norse gods.
In Norse mythology, Freya is a goddess of love and fertility, and the most beautiful and propitious of the goddesses. She is the patron goddess of crops and birth, the symbol of sensuality and was called upon in matters of love. She loves music, spring and flowers, and is particularly fond of the elves (fairies). Freya is one of the foremost goddesses of the Vanir.How can you not love a god who is fond of eleves?
Where has Freya gone now that nobody believes in her any more? Did she die? Is she buried somewhere?
These are important questions because there are hundreds of extinct gods and we don't know what's happened to them. Jerry Coyne tries to come up with an answer [Where are all the dead gods?] but I fear that his knowledge of religion isn't sufficient for such a complex topic.
Maybe we should ask one of those sophisticated Christians that we hear so much about?
Do you remember William Lane Craig? [Why Reasonable People Should Not Debate William Lane Craig] He tried to educate Jerry Coyne about science and religion. Listen to his broadcast and see how Jerry responds at: William Lane Craig goes after me for ignorance of religion and science.
Don't you just love it when those sophisticated Christians teach us about sophisticated science? Perhaps he would do better if he got another degree. So far he only has a Ph.D. (Philosophy) and a Doctor of Theology (Th.D.). Poor old Jerry Coyne only has a single Ph.D. (under Richard Lewontin). Jerry is completely outclassed in the sophisticated department but I bet he wears better boots!
Be a Proud Atheist
 We Are Atheism Campaign from the Richard Dawkins Foundation.
We Are Atheism Campaign from the Richard Dawkins Foundation.
This is your chance to finally be heard. This is our chance to stand up, speak out, and be counted. We want to provide a platform for atheists around the globe to see that they are not alone. Atheists come in all shapes, sizes, ages, and backgrounds. The only thing that we all have in common is that we don’t see any credible evidence to believe in a god. It’s ok to be an atheist, and we want the world to know.We are not just here to let you watch movies; these are real people living real lives as atheists. We want the world to know we exist and we will not be ignored. We will stand up, speak out, and be counted.
- Provide an outlet for atheists to feel comfortable to come out of the closet.
- Always let visitors know there are other people out there that are non-believers.
- Help people find other atheists like them in their state, city, and even neighborhood.
- Give access to local, national, and international organization to become involved in the secular community.
- Empower people to start their own organization in areas that does not already have one.
Labels:
Atheism
Despicable Rhett S. Daniels
EpiRen is the pseudonym of a blogger who about public health issues, including vaccinations and various forms of quackery. He works for a state public health department in the United States. At some point EpiPen crossed paths with Rhett S. Daniels and Daniels didn't like what he heard.
So what did Daniels do? He had EpiPen investigated and "outed" him to his employers. The employers told EpiPen to stop blogging about these issues or be fired. EpiPen complied—as we all would under such circumstances. Read all about it at: The consequences of blogging under one's own name] [A Public Servant, Blogging and Tweeting Under His Own Name, Has Been Silenced By His Employers].
Rhett Daniels showed up in the comments section on the second blog and started issuing more threats.
i am mr. x; first, i am not anti-vax; second, i didn't want epiren to stop posting, but rather to take down the defamatory blog; third, i am not done going after every individual who defames me.Who is this despicable person? It's a little tricky to find out since he's in the process of erasing his blog, his twitter account, and several other internet references. His YouTube videos on folate have been made inaccessible and the websites of some of his companies seem to be undergoing routine maintenance. But there are still traces of him on the internet as PZ Myers discovered: Rhett S. Daniels, litigious bully.
you think you are safe, but all i have to do is file a john doe - or hire a cyber investigator. these courses of action cost less than $10,000 each; which means every person who is afraid of the light can be exposed.
i will not tolerate harassment, defamation, or any such action by any of you. i am very aware of all of you, and have the capital and the will to go after each and every one of you ONLY IF you defame or slander me.
i am self employed if you count owning 11 pharmaceutical companies with cum gross sales over 1/2 billion.
....
actuall, to save me $9,000 i will offer $1,000 for identify info that leads to an address where i can serve anarchic teapot (legally serve as in sheriff delivers court papers).
I'm doing my bit to create an internet presence for Rhett Daniels—I'm sure he'll thank me when he gets a chance. Let me make it clear that I am not slandering Rhett Daniels. I'm merely stating what the evidence strongly suggests; namely, that he is a despicable, cowardly, bully who will use his money to legally harass anyone who dares to criticize his treatments.
Bits and pieces of his blog are still accessible on Google cache: cigaRHETT - Toxicological Insight. From there you can link to his Blogger profile where you discover that his favorite movie was Top Gun and one of his two favorite books is the Bible. (Why are we not surprised that such a despicable man would like the Bible?) He lives in Fort Myers, Florida, United States.
Here's more,

Activist, philanthropist and entrepreneur. Highly skilled executive who excels at taking small struggling companies from low to high revenues in short periods. Boast an impressive record of the most pharmaceutical drug products ever developed by one person (over 400) in the history of pharma - surpassing my idol, Robert Stockstad from Lederle Pharma (he developed folic acid in 1947). One of the most successful non-lawyer ProSe litigants in history. As of July 18, 2011, total product sales since July 18, 2006, are: $590,635,984 (and 22,728,724 units sold!). politics: www.linkedin.com/in/RhettSDaniels ViaDiem Holdings (Founder): www.viadiem.com Captiva Pharma (CEO): www.CaptivaRx.com Goals: (1) To lead a small pharma company from less than $20 million/yr to over $1 billion/yr in revenues; (2) To feed 82 million Africans with my new unique ingredients that purifies water while provided demographic and staple-deficient based vitamins to provide min RDA based on regional disparities;Rhett Daniels seems to have forgotten that there are some things that are both wrong and evil.
You get to ride the big roller coaster three times in a row. What will keep your dad from taking a bite out of your candy apple?
Doing the Good versus doing the Right. Somethings can be Right that are not Good; and there are many things that are Good that are not Right. Right means legally, and Good means natural law.
There are several things wrong here. Daniels behaved badly by taking the disagreement to EpiPen's employer. The employer behaved badly by threatening EpiPen if he didn't stop blogging. We should aim for a society where neither of those behaviors are acceptable and everyone can speak freely without fear of retaliation. This is not a good time to criticize the employers but we can make sure Rhett Daniels appreciates the consequences of his behavior. Judging by his defensive reactions on the internet, I think he might be learning a lesson ....
UPDATE: Read the "warning letter" that Liz Ditz received from Rhett Daniels. If this weren't so sad it would be funny.
Here's a list of posts on Keeping Up with #EpiGate. Lots of people are trying to help Rhett S. Daniels have a visible presence on the internet. He will be very grateful.
Labels:
Blogs
Sunday, August 21, 2011
Chitty Chitty Bang Bang Bang Bang

We recently learned that Prager University was instrumental in converting GilDodgen from obnoxious atheist to obnoxious theist [How to Convince an Atheist to Become an IDiot]. GilDodgen liked to two "courses" in particular. One was a "course" on "The Most Important Verse in the Bible." All five minutes of the course were taught by Dennis Prager.
The other "course" is taught by Frank Pastore, a former atheist and a former baseball player. He's now the host of a Christian talk show in Los Angeles. Pastore is a high school graduate. (Pastore was "converted" by some other Cincinnati Reds baseball players.)
You need to watch this video. Here's how it's described in the Prager University calendar.
Who takes the greater leap of faith -- the atheist or the believer? Best selling author and award-winning radio talk show host, Frank Pastore, poses this question in this compelling Prager University video course.
WARNING: Parts of this video may be harmful to the rational mind. Viewer discretion is advised.
How Does Something Get into a Textbook?
 A recent paper in Molecular Cell involved the study of nucleosome assembly in vitro (Torigoe et al. 2011). The authors were looking for intermediate stages in the assembly of nucleosome after DNA replication. Here's the abstract of their paper ...
A recent paper in Molecular Cell involved the study of nucleosome assembly in vitro (Torigoe et al. 2011). The authors were looking for intermediate stages in the assembly of nucleosome after DNA replication. Here's the abstract of their paper ...
Chromatin assembly involves the combined action of histone chaperones and ATP-dependent motor proteins. Here, we investigate the mechanism of nucleosome assembly with a purified chromatin assembly system containing the histone chaperone NAP1 and the ATP-dependent motor protein ACF. These studies revealed the rapid formation of a stable nonnucleosomal histone-DNA intermediate that is converted into canonical nucleosomes by ACF. The histone-DNA intermediate does not supercoil DNA like a canonical nucleosome, but has a nucleosome-like appearance by atomic force microscopy. This intermediate contains all four core histones, lacks NAP1, and is formed by the initial deposition of histones H3-H4. Conversion of the intermediate into histone H1-containing chromatin results in increased resistance to micrococcal nuclease digestion. These findings suggest that the histone-DNA intermediate corresponds to nascent nucleosome-like structures, such as those observed at DNA replication forks. Related complexes might be formed during other chromatin-directed processes such as transcription, DNA repair, and histone exchange.Interesting but hardly Earth-shattering. More work needs to be done to confirm this result and see if it's significant in vivo. At least that's what you would think if you just looked at the paper.
You get a very different perspective if you read the press release from the University of California at San Diego: Biologists' Discovery May Force Revision of Biology Textbooks: Novel Chromatin Particle Halfway Between DNA and a Nucleosome.1
Basic biology textbooks may need a bit of revising now that biologists at UC San Diego have discovered a never-before-noticed component of our basic genetic material.
According to the textbooks, chromatin, the natural state of DNA in the cell, is made up of nucleosomes. And nucleosomes are the basic repeating unit of chromatin.
 That's correct. All the textbooks have a diagram similar to the one shown here from my textbook. It shows the organization of nucleosome core particles and the completed nucleosome on DNA.
That's correct. All the textbooks have a diagram similar to the one shown here from my textbook. It shows the organization of nucleosome core particles and the completed nucleosome on DNA.
What the new result shows is that there's an intermediate stage where the core particle is bound to DNA but the DNA isn't wrapped around the core particle. That's not a big surprise and it's not going to make it into most textbooks, even if it's true.
"This novel particle was found as a precursor to a nucleosome," said James Kadonaga, a professor of biology at UC San Diego who headed the research team and calls the particle a "pre-nucleosome." "These findings suggest that it is necessary to reconsider what chromatin is. The pre-nucleosome is likely to be an important player in how our genetic material is duplicated and used."This is mostly hype and none of this speculation is found in the actual paper. Unfortunately, this sort of press release has become the norm and that's got to stop.
The biologists say that while the pre-nucleosome may look something like a nucleosome under the microscope, biochemical tests have shown that it is in reality halfway between DNA and a nucleosome.
These pre-nucleosomes, the researchers say, are converted into nucleosomes by a motor protein that uses the energy molecule ATP.
"The discovery of pre-nucleosomes suggests that much of chromatin, which has been generally presumed to consist only of nucleosomes, may be a mixture of nucleosomes and pre-nucleosomes," said Kadonaga. "So, this discovery may be the beginning of a revolution in our understanding of what chromatin is."
This work isn't even close to making into the textbooks for a number of reasons. The most obvious is that it needs to be confirmed. Textbook writers do not immediately put new findings into their books because we've been burned too many times. But there's another reason why this ain't gonna make it—it's not important enough.
Textbooks are not encyclopedias. They will only contain information that undergraduates need to know in order to understand the basic concepts and principles in the field. I know that every scientist thinks his or her most recent discovery is Nobel Prize work and I'm sure they would like every biochemistry undergraduate to know about it. At some point a textbook author has to decide what's really important and, unfortunately, those choices mean that 99.99% of everything that's published in a given year doesn't make the cut.
It can't be any other way.
1. It even seemed important enough for Richard Dawkins.net: Biologists' Discovery May Force Revision of Biology Textbooks: Novel Chromatin Particle Halfway Between DNA and a Nucleosome.
How to Convince an Atheist to Become an IDiot
 You all remember GilDodgen, right? He's one of the IDiots who post regularly on Uncommon Descent. Nothing that he says about Intelligent Design Creationism is unusual but he does have one characteristic that appeasr to set him apart. Here's how he describes himself [ID and Prager University].
You all remember GilDodgen, right? He's one of the IDiots who post regularly on Uncommon Descent. Nothing that he says about Intelligent Design Creationism is unusual but he does have one characteristic that appeasr to set him apart. Here's how he describes himself [ID and Prager University].
As many UD readers know, I was once a Richard Dawkins-style atheist. I was not just an ordinary, garden-variety atheist, but a really obnoxious, nasty, self-aggrandizing, pathetically prideful atheist like Dawkins. I prided myself in using my intellectual capacities in an attempt to destroy any belief that materialism cannot explain everything.Can you believe it? He used to be just like Richard Dawkins: obnoxious, nasty, self-aggrandizing, and prideful. (GilDodgen is still all of those things but now he's an IDiot.)
What an amazing transformation! I bet you're wondering, just like me, how the other IDiots managed to convert him.
Well wait no longer 'cause GilDodgen lets us in on the secret.
What a fool I was. The story of my conversion is available, but the most salient point concerning ID is that my interest and expertise in basic science, engineering, and especially highly sophisticated computational algorithms, led me to recognize the inherent design in living systems and the transparent desperation of ID opponents to explain away the obvious.Did you resist clicking on the links to Prager University? No, neither did I. There was just too much potential for a good laugh.
A major influence in my journey over the years has been Dennis Prager. I first started listening to him on the radio more than 20 years ago. His intelligence, eloquence, and articulation about ultimate issues had a profound effect on me.
Prager is a Jew, not a mindless evangelical Christian.
For those who are interested, check out Prager University, especially here and here.
Let's look at the first of these major influences on the life of an atheist. We'll save the other one for later [Chitty Chitty Bang Bang Bang Bang].
The first thing you notice about the video is the title: The Most Important Verse in the Bible. That's exactly the sort of thing a nasty, materialist, atheist might be watching, right? Of course it is. That's exactly why we're all going to watch it!

The second thing you notice is the cation under the video.
No one, not even the most devoted atheist, denies that the Bible is the most influential book ever written. So, what is the most important verse in this most important book?Now correct me if I'm wrong, but aren't there five billion non-Christians on this planet? Is it true that they can't think of a more influential book among all those that have ever been written?
Really? I didn't know that.
Before you watch the video, see if you can think of the most important verse in the Bible—assuming you have read it. Now watch the video and see how convincing it would be for a typical atheist.
WARNING: This video contain powerful theistic messages. Watching it might be hazardous to the rationality of atheists. Viewer discretion is advised.1
1. For the benefit of all non-Americans I should explain that this phrase is prominently displayed before every segment of a TV show (or movie) where you might catch a glimpse of an uncovered female breast or hear the word "shit." It's got to be one of the stupidest, meaningless, sentences every written.
Saturday, August 20, 2011
Protein Folding, Chaperones, and IDiots
 We know a lot about protein synthesis and structure. Proteins are made by the translation machinery (ribosomes + factors) as they copy the information in messenger RNA. When they are first synthesized, proteins can be visualized as random coils or even linear molecules consisting of a long string of amino acids joined end-to-end.
We know a lot about protein synthesis and structure. Proteins are made by the translation machinery (ribosomes + factors) as they copy the information in messenger RNA. When they are first synthesized, proteins can be visualized as random coils or even linear molecules consisting of a long string of amino acids joined end-to-end.
Eventually these newly synthesized molecules have to fold into a specific three-dimensional shape that's different for every protein. The diagram on the right illustrates this process for some hypothetical folding pathways.
To a first approximation, the final three-dimensional shape is determined by the amino acid sequence of the protein. The final shape represents the lowest free energy state of the folded protein and this can be represented as a free energy well. Left to their own devices, almost all proteins will eventually reach the bottom of the deepest well that represents the functional state of the protein. (There are exceptions to every rule in biology but this is a very good generality.)
 There are many dips and troughs in the free energy landscape and sometimes proteins get trapped in a local minimum as shown by path B in the diagram on the left. If you wait long enough, the incorrectly folded protein will eventually get out of the local dip and fold into the correct shape. (This depends on an energy of activation.)
There are many dips and troughs in the free energy landscape and sometimes proteins get trapped in a local minimum as shown by path B in the diagram on the left. If you wait long enough, the incorrectly folded protein will eventually get out of the local dip and fold into the correct shape. (This depends on an energy of activation.)
For the majority of proteins, this spontaneous folding is quite rapid. They reach the proper three-dimensional structure in seconds or minutes. For some proteins it may take much longer, especially if the free energy landscape is rugged and has many deep pits. When a spontaneous biochemical reaction is too slow to be useful it usually means that an enzyme is required to speed up the reaction. Recall that the role of enzymes is to accelerate reactions that occur spontaneoulsy—they do not create new reactions.
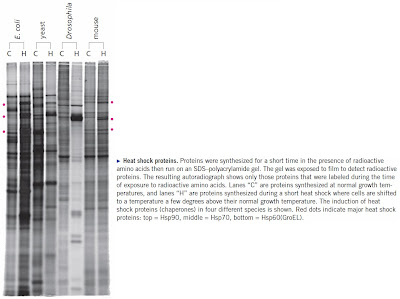
The "enzymes" that speed up protein folding are called molecular chaperones and they are among the most highly conserved enzymes in all of biology. As you might expect, these ancient enzymes are present in all species. There are several different kinds of chaperones but one of the most common is called HSP70 (heat shock protein of 70kDa). [Heat Shock and Molecular Chaperones] [The Evolution of the HSP70 Gene Family] [Gene HSPA5 Encodes BiP-a Molecular Chaperone].
HSP70 binds to hydrophobic regions of the folding protein preventing it from aggregating with other partially folded proteins and steering it toward the final three-dimensional structure. This greatly speeds up the folding pathway for those proteins that are otherwise slow to fold. Obviously there has been selection for rapidly folding proteins and/or selection for those that can be effectively assisted by chaperones. The genes for other proteins have not survived so what we see today are proteins that can fold rapidly with, or without, the assistance of chaperones.
Ulrich Hartl has just published a nice review of chaperones in Nature (Hartl et al. 2011). It didn't take long for the IDiots to comment. I spotted a posting on Uncommon Descent:Nature Review Article Yields Unpleasant Data For Darwinism, but that's just a link to another blog posting by a British IDiot named Antony Latham: New research on protein folding demonstrates intelligent design. Here's what Antony Latham has to say about chaperones.
The review in the journal Nature does not discuss the origins of these systems but we need to ask a question: how does all this fit with current evolutionary theory? One might think that such complex systems are confined to mammals or at least the higher orders of animals. This would be a mistake however, because chaperones and chaperonins are in bacteria and archaea also. Indeed it would seem that for any cell to function there needs to be not just proteins but, at the same time, these chaperone systems, which are absolutely essential for proper folding and maintenance of proteins. Without such systems, in place already, the cell will not function.All of the common chaperones fold spontaneously without the assistance of any other chaperones. The reason why they are called "heat shock" proteins is because their synthesis is induced when cells encounter high temperature or other conditions that may cause proteins to unfold or become unstable. These rescue chaperones are made in huge quantities under these conditions to help prevent the destruction of normal cellular proteins. If you understand this then you will understand that the chaperones themselves are capable of rapid spontaneous folding. Even if you didn't know the facts this would seem obvious.
Now, as explained, these chaperone systems are themselves made of proteins which also require the assistance of chaperones to correctly fold and to maintain integrity once folded. Chaperones for chaperones in fact. The very simplest of cells that we know of have these systems in place.
Darwinian evolution requires step by step changes in molecular systems, with one step leading to another in a manner that is statistically reasonable to expect from selection of mutant strains. There is no Darwinian explanation however for the evolution of proteins which already have chaperone systems in place to ensure proper function.
This points very strongly to an intelligent origin of these ‘ingenious’ systems found in all of life.
In the beginning, you didn't need chaperones because every protein folded rapidly on its own. Some of these primitive proteins might have been a bit slow to fold so the evolution of the first chaperones was advantageous because it enhanced the rate of folding for these proteins. The chaperones weren't absolutely necessary for survival but they conferred a selective advantage on those cells that had them.
Once chaperones were present, new proteins could evolve that would otherwise have been too slow to fold in the absence of chaperones. Over time, cells accumulated more and more of these slowly folding proteins so that today no cell can survive without chaperones.
What we can't explain is why the IDiots keep putting their foots in their mouths.
Hartl, F.U., Bracher, A., and Hayer-Hartl, M. (2011) Molecular chaperones in protein folding and proteostasis. Nature 475: 324–332. [Nature]
Ron Paul doesn't believe the theory, but what about the fact?
Here's a video where Ron Paul proclaims that he doesn't believe the Theory of Evolution. Unfortunately there's no followup question to find out whether he believes the facts of evolution. Why can Ron Paul make such a nonsensical statement and still be considered as someone who might be the "Leader of the Free World" (sic)? It's because of decades of brainwashing by creationists who blatantly ignore the science behind evolutionary biology and the distinction between fact and scientific theory.
I'm trying to understand the behavior of the creationists. It seems to me that there are only two possibilities; either they are completely stupid and ignorant or they are lying. There doesn't seem to be any other explanation. In the case of the most prominent creationists, I'm inclined to believe they are lying because I know for a fact they've read up on Evolution Is a Fact and a Theory.
This doesn't apply to Ron Paul, by the way. I don't think he's lying.
[Hat Tip: Pharyngula: Ron Paul gets no respect. I think this is a video from 2008.
Nobel Prize in ... 2060
Friday, August 19, 2011
Evolution of a New Enzyme
 The evolution of new genes and their new enzymes often takes place after a gene duplication event followed by the adaptation of one or both of the duplicated enzymes to a particular substrate.
The evolution of new genes and their new enzymes often takes place after a gene duplication event followed by the adaptation of one or both of the duplicated enzymes to a particular substrate. Most people think of enzymes as being highly specific for a particular substrate so they see this adaptation as an all-or-none affair involving a fairly drastic change in substrate binding. Creationists, in particular, are prone to this mistaken view of biochemistry so they see the evolution of a new enzyme activity as a difficult process. Sometimes they note that several amino acid substitutions are required to change substrates and they declare that this is beyond the reach of gradual evolution.
I'm going to tell you about the evolution of a new enzyme that has been caught in progress. This example will help you realize that enzymes don't have to be highly specific for a single substrate and it will help you appreciate how easy it is to evolve new substrate specificities from sloppy precursors. That's the way it probably happens in most cases.
The top diagram shows the pathways of biosynthesis of three amino acids that have branched side chains: isoleucine, valine, and leucine. Bear with me for a minute while I explain the chemistry—it will be worth it in the end.
In order to make isoleucine you need to combine two simple molecules, pyruvate and α-ketobutyrate, to make a molecule with seven carbon atoms. This molecule then undergoes three reaction to produce the six-carbon molecule α-keto-β-methylvalerate (with the loss of CO2). Now look at the pathway for the synthesis of valine and leucine (on the right). The first step combines a molecule of pyruvate with another molecule of pyruvate to produce a six-carbon compound that is subsequently converted to the five-carbon compound α-ketoisovalerate.
 The neat thing about these two pathways is that the first four steps are catalyzed by the same four enzymes! Each of these enzymes is capable of recognizing two different substrates.
The neat thing about these two pathways is that the first four steps are catalyzed by the same four enzymes! Each of these enzymes is capable of recognizing two different substrates. For example, the first enzyme is acetohydroxy acid synthase. It can combine a pyruvate molecule with another pyruvate molecule or with α-ketobutyrate. Both reactions are catalyzed at high efficiency. The normal enzyme has a preference for α-ketobutyrate as the acceptor molecule because the concentration of α-ketobutyrate inside cells is much lower than the concentration of the donor molecule, pyruvate. This preference ensures that the rate of synthesis of isoleucine is comparable to synthesis of valine and leucine.
There are two similar genes for acetohydroxy acid synthase in some bacteria. They clearly arise from a recent gene duplication event. One of the genes encodes an enzyme with the standard preference for α-ketobutyrate but the other encodes an enzyme that prefers to combine two molecules of pyruvate. Both enzymes (AHAS I and AHAS II) catalyze both reactions but they differ in their preference for the acceptor substrate (Epelbaum et al. 1998, Steinmetz et al. 2010).
 Under normal growth conditions (glucose as a carbon source) in Salmonella typhimurium, the typical enzyme that prefers α-ketobutyrate as acceptor (ASAS II) is all that's required. (You can knock out the other gene and growth isn't affected.) However, the other isozyme (ASAS I) is essential when the cells are grown on acetate as the sole carbon source. This is because in the presence of acetate the internal concentration of pyruvate is low so you need an enzyme that binds both acceptor molecules equally well.
Under normal growth conditions (glucose as a carbon source) in Salmonella typhimurium, the typical enzyme that prefers α-ketobutyrate as acceptor (ASAS II) is all that's required. (You can knock out the other gene and growth isn't affected.) However, the other isozyme (ASAS I) is essential when the cells are grown on acetate as the sole carbon source. This is because in the presence of acetate the internal concentration of pyruvate is low so you need an enzyme that binds both acceptor molecules equally well. What we have here is an example that helps us understand how homologous enzymes may have evolved in the ancient past. The ancestral enzyme was capable of catalyzing several similar reactions. Following a gene duplication event, the two separate genes evolved independently to specialize in just one of the reactions that the original enzyme could catalyze. Neither of them had to evolve an entirely new substrate binding site, they only had to hone an already existing site.
The important observation is that not all enzymes are highly specific. Even modern enzymes that catalyze common reactions can be shown to catalyze similar reactions at a low level. The important conceptual point is that ancient enzymes were certainly very sloppy and frequently catalyzed a wide range of similar reactions. This is what you expect during early evolution. You don't expect highly efficient, highly specific, enzymes to just pop into existence out of the blue.
The take-home lesson is that the evolution of two homologous enzymes that catalyze different, but similar, reactions did not arise by switching from one activity to another. Instead, they arose from a common ancestor that could catalyze both reactions.
[The pathways are from Moran et al. (2012) Principles of Biochemsitry and the enzyme is acetohydroxy acid synthase from yeast (PDB=1T9C)]
Epelbaum, S., LaRossa, R.A., VanDyk, T.K., Elkayam, T., Chipman, D.M., and Barak, Z. (1998) Branched-chain amino acid biosynthesis in Salmonella typhimurium: a quantitative analysis. J. Bacteriol. 180:4056-4067. [PubMed Central]
Steinmetz, A., Vyazmensky, M., Meyer, D., Barak, Z.E., Golbik, R., Chipman, D.M., and Tittmann, K. (2010) Valine 375 and phenylalanine 109 confer affinity and specificity for pyruvate as donor substrate in acetohydroxy acid synthase isozyme II from Escherichia coli. Biochemistry 49:5188-5199. [PubMed]
Reading Books
 Now that I've finished writing my book, I'm back into reading. I have a pile of books that I have to get through before classes start. It's going to be difficult 'cause I'm off to Brussels next week to visit my granddaughter Zoë.
Now that I've finished writing my book, I'm back into reading. I have a pile of books that I have to get through before classes start. It's going to be difficult 'cause I'm off to Brussels next week to visit my granddaughter Zoë.
I mostly read non-fiction with an emphasis on science, philosophy, history, theology, and creationism. When I'm finished with a book it's usually full of highlighted text and margin notes and many of the pages have sticky tags for quick reference. Every single one of my books becomes part of my reference library and I almost always consult them again after reading.
I can't imagine how anyone like me could ever make use of an electronic reader. I've got exactly three books on my iPad (Pride and Prejudice, Treasure Island, and Aesop's Fables) and that's only because they came with the kindle app. I will never read them.
A couple of days ago I discovered another thing you can do with a real book (paperback) that you can't do with a kindle or other reader—especially an expensive iPad. It was a horrible book that I had just finished and it felt really, really, good to throw it across the room into the waste basket. I retrieved it later on for future reference but the gesture was immensely satisfying.
[Photo credit: My daughter flew in from Brussels a few days ago on her way to Newark. She had to take care of some business in Toronto so she stayed the night in her old room. I discovered this little scene on her bed after she had left.]
Labels:
Books
,
Science
,
Science Education
Here Be Dragons
 I first met Stefaan Blancke (left) and Maarten Boudry (right) when they came to Toronto for a conference in November, 2009. A few months later I visited Maarten at the University of Gent in Belgium (Stefaan wasn't there on the day I visited) [Good News from Gent].
I first met Stefaan Blancke (left) and Maarten Boudry (right) when they came to Toronto for a conference in November, 2009. A few months later I visited Maarten at the University of Gent in Belgium (Stefaan wasn't there on the day I visited) [Good News from Gent]. These young philosophers presented a paper on Methodological Naturalism that impressed me enormously. The paper was eventually published in June 2010 [Methodological Naturalism - How Not to Attack Intelligent Design Creationism].
 The essence of their paper is that science is not intrinsically limited to methodological naturalism in spite of what many people—especially accommodationists—might say. (And in spite of what was said in court in Dover, Pennsylvania.) Boudry and Blanke (and Johan Braeckman) claim that science is perfectly capable of investigating supernatural claims. However, whenever scientists have done this they have discovered that the claims are either false or unsupported by evidence. Hence, science is characterized by "provisory" methodological naturalism based on empirical evidence. This is very different from "intrinsic" methodological naturalism.
The essence of their paper is that science is not intrinsically limited to methodological naturalism in spite of what many people—especially accommodationists—might say. (And in spite of what was said in court in Dover, Pennsylvania.) Boudry and Blanke (and Johan Braeckman) claim that science is perfectly capable of investigating supernatural claims. However, whenever scientists have done this they have discovered that the claims are either false or unsupported by evidence. Hence, science is characterized by "provisory" methodological naturalism based on empirical evidence. This is very different from "intrinsic" methodological naturalism. Maarten Boudry has written lots more about pseudoscience in general and Intelligent Design Creationism in particular. It's all published in his thesis: Here Be Dragons. I suggest you read the whole thing!
[Image Credit: rbh.Smaug.jpg. Smaug is from The Hobbit. It's also the favorite dragon of my colleague Craig Smibert who discovered the Smaug (Smg) gene/protein in Drosophila melanogaster.]
Physicists and Biologists
 I've just finished reading evolution: a view from the 21st century by James A. Shapiro. I'll write up a full review later on but right now I just want to quote a passage from near the end of the book. He begins the paragraph with a description of those physicists who entered biology in the 1940s and 50s (e.g. Max Delbrück).
I've just finished reading evolution: a view from the 21st century by James A. Shapiro. I'll write up a full review later on but right now I just want to quote a passage from near the end of the book. He begins the paragraph with a description of those physicists who entered biology in the 1940s and 50s (e.g. Max Delbrück). Currently another wave of physical scientists is entering the life sciences. They bring with them a much-needed and fruitful sophistication in observation at the micro level, in mathematical formulation of results, and in computational methods of data analysis. Physicists-turned-biologists have an additional advantage of lacking a formal education in the life sciences; consequently, they have not been taught to exclude from their thinking notions previously concluded to be "impossible." We can only hope that their less prejudiced backgrounds will make it easier for them to develop novel conceptual frameworks to complement the analytical and experimental techniques they are introducing.This insightful observation has great potential beyond solving the major problems in the biological sciences and I wonder if Shapiro fully appreciates the implications.
I have no formal training in physics. I haven't the foggiest idea what quantum chromodynamics (QCD) is all about, beyond what I can read on Wikipedia. I don't have a firm grasp of general relativity and my math skills are very weak.
However, I understand that physics is grappling with unified field theory and that string theory is going nowhere. I've heard rumors that physicists can't find the Higgs boson, although I can't imagine where they might have put it. I have plenty of experience helping Ms. Sandwalk find her car keys and credit card so I've come up with a brilliant idea.
Why don't I move to physics and solve their problems? I've got all the proper qualifications, "lacking a formal education," "less prejudicial background," and I haven't been taught to exclude impossible things. I bet I could convince half a dozen of my biologist colleagues to abandon the difficult problems of biology in order to help the physicists. It shouldn't take more than a few years.
We need a name for this discovery, let's call it The Shapiro Conjecture.1
Meanwhile, I welcome all those physicists who know nothing about evolution, protein structure, genetics, physiology, metabolism, and ecology. That's just what we need in the biological sciences to go along with all the contributions made by equally ignorant creationists.
AFTERTHOUGHT: Biologists have been using computers to analyze complex data sets for over fifty years and we're pretty sophisticated at making observations at the micro level. Why do we need physicists to show us these techniques?
1. See The Salem Conjecture.
Labels:
Science
Magical Mirrors
 Sometimes it's fun to set aside trivial questions like evolution vs. creationism and address the really important questions in life. The last time we did this was when we discussed the proper way to hang toilet paper [Gil Dodgen Explains the Salem Conjecture].
Sometimes it's fun to set aside trivial questions like evolution vs. creationism and address the really important questions in life. The last time we did this was when we discussed the proper way to hang toilet paper [Gil Dodgen Explains the Salem Conjecture].
Chad Orzel of Uncertain Principles has posted a link to Rhett Allain at DOT.PHYSICS who asks one of those big questions that we've all pondered obsessively ...
Why do mirrors reverse left and right, but they don’t reverse up and down?.Please proceed with caution because Rhett comes about as close as one can to answering the question—thus removing it from the top ten list of mysteries. If you want to preserve your childhood fantasies about the magical properties of mirrors then I advise you to ignore this posting.
Subscribe to:
Comments
(
Atom
)

