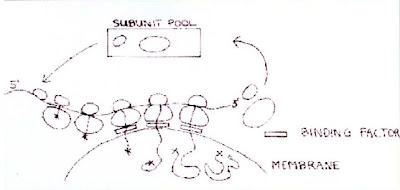
The signal recognition particle binds to ribosomes at the site of the exit tunnel and interacts with the N-terminal end of newly synthesized protein. If the protein contains a signal sequence then protein synthesis is temporarily arrested. The complex is directed to the membrane surface and the end of the protein is inserted through a pore in the membrane. Protein synthesis then continues and the newly synthesized protein is inserted into the endoplasmic reticulum in eukaryotes or across the plasma membrane in bacteria [
The Signal Hypothesis].

In all species (bacteria and eukaryotes) the signal recognition particle is composed of a conserved ~300 nucleotide RNA molecule (7SL/7S RNA) and several (usually six) proteins. Only one of the proteins is common to both bacteria and eukaryotes (Hainzl et al. 2007). The organization of the mammalian SRP is shown above (Maity and Weeks, 2007).

There are two distinct regions of SRP. The large subunit consists of nucleotides 101-255 of the 7S RNA molecule. The RNA is folded into the secondary structure shown on the left (Mauty and Weeks, 2007). In this diagram, the linear nucleotide sequence is connected by solid lines with arrows and the dashed lines depict hydrogen bond interactions between bases that are brought into proximity by the secondary, and tertiary, structure of the RNA molecule.
Four proteins (SRP19, SRP54, SRP68, SRP72) are bound to the large subunit. SRP54 is the only protein found in all species. The large subunit recognizes the signal peptide and binds to the membrane receptor.
The other subunit is called the Alu subunit. The significance of this name will become clear in another posting. The RNA component consists of nucleotides 1-100 and 256-299 arranged in a single double helical structure. Proteins SRP9 and SRP14 bind to the Alu subunit. The Alu subunit is responsible for arresting protein synthesis.
SRP is an important cellular component and it plays a crucial role in protein synthesis and protein trafficing. It's also a good example of a ribonucleotide particle present in all species. Most people don't appreciate the fact that many small RNAs are an integral part of the cellular machinery. Other examples are the telomerase complex and RNAse P. These small RNAs have been known for a long time. The recent publicity about other types of small RNAs has unfortunately conveyed the impression that this is a new discovery.
The structure of SRP has not been fully worked out but a great deal is known about the various components. A model of the complete mammalian signal recognition particle, incorporating the known structures of the RNA and the various proteins, is shown below (Halic and Beckmann, 2005).

Hainzl, T., Huang, S. and Sauer-Eriksson, A.E. (2007) Interaction of signal-recognition particle 54 GTPase domain and signal-recognition particle RNA in the free signal-recognition particle.
Proc. Natl. Acad. Sci. (USA) 104:14911-6. [PubMed]
Halic, M. and Beckmann, R. (2005) The signal recognition particle and its interactions during protein targeting. Curr. Opin. Struct. Biol. 15(1:116-125. Review. [PubMed]
Maity, T.S. and Weeks, K.M. (2007) A threefold RNA-protein interface in the signal recognition particle gates native complex assembly. J. Mol. Biol. 369:512-24 [PubMed]
 From CNN.com comes scary news about our southern border [ Report: Security on U.S.-Canada border fails terror test].
From CNN.com comes scary news about our southern border [ Report: Security on U.S.-Canada border fails terror test].