 I just found a new website called the The Humanist Globe. It was created by Mark Robinson who I met at the opening of the Centre for Inquiry (Toronto) last year.
I just found a new website called the The Humanist Globe. It was created by Mark Robinson who I met at the opening of the Centre for Inquiry (Toronto) last year. Check it out. It's full of all kinds of interesting things.
 I just found a new website called the The Humanist Globe. It was created by Mark Robinson who I met at the opening of the Centre for Inquiry (Toronto) last year.
I just found a new website called the The Humanist Globe. It was created by Mark Robinson who I met at the opening of the Centre for Inquiry (Toronto) last year. 
Figure 4.10The α-helical conformation was proposed in 1950 by Linus Pauling and Robert Corey. They considered the dimensions of peptide groups, possible steric constraints, and opportunities for stabilization by formation of hydrogen bonds. Their model accounted for the major repeat observed in the structure of the fibrous protein α-keratin. This repeat of 0.50 to 0.55 nm turned out to be the pitch (the axial distance per turn) of the α helix. Max Perutz added additional support for the structure when he observed a secondary repeating unit of 0.15 nm in the X-ray diffraction pattern of α-keratin. The 0.15 nm repeat corresponds to the rise of the helix (the distance each residue advances the helix along its axis). Perutz also showed that the α helix was present in hemoglobin, confirming that this conformation was present in more complex globular proteins.
α Helix. A region of α-helical secondary structure is shown with the N-terminus at the bottom and the C-terminus at the top of the figure. Each carbonyl oxygen forms a hydrogen bond with the amide hydrogen of the fourth residue further toward the C-terminus of the polypeptide chain. The hydrogen bonds are approximately parallel to the long axis of the helix. Note that all the carbonyl groups point toward the C-terminus. In an ideal α helix, equivalent positions recur every 0.54 nm (the pitch of the helix), each amino acid residue advances the helix by 0.15 nm along the long axis of the helix (the rise), and there are 3.6 amino acid residues per turn. In a right-handed helix, the backbone turns in a clockwise direction when viewed along the axis from its N-terminus. If you imagine that the right-handed helix is a spiral staircase, you will be turning to the right as you walk down the staircase.

Figure 4.11
View of a right-handed α helix. The blue ribbon indicates the shape of the polypeptide backbone. All the side chains, shown as ball-and-stick models, project outward from the helix axis. This example is from residues Ile-355 (bottom) to Gly-365 (top) of horse liver alcohol dehydrogenase. Some hydrogen atoms are not shown. [PDB 1ADF].
Horton, H.R., Moran, L.A., Scrimgeour, K.G., perry, M.D. and Rawn, J.D. (2006) Principles of Biochemisty. Pearson/Prentice Hall, Upper Saddle River N.J. (USA)

 In 1954, Linus Carl Pauling (1901 - 1994) was awarded the Nobel Prize in Chemistry for his work on the nature of chemical bonds and the structures of complex molecules. In the field of biochemistry he is best known for working out the form of the α helix in 1948 (published in 1950). The α helix is a stretch of secondary structure in proteins where the polypeptide chain forms a helix. The theoretical model was quickly confirmed by Perutz's group at Cambridge where Francis Crick was working at the time [see The Storyof DNA: Part 1].
In 1954, Linus Carl Pauling (1901 - 1994) was awarded the Nobel Prize in Chemistry for his work on the nature of chemical bonds and the structures of complex molecules. In the field of biochemistry he is best known for working out the form of the α helix in 1948 (published in 1950). The α helix is a stretch of secondary structure in proteins where the polypeptide chain forms a helix. The theoretical model was quickly confirmed by Perutz's group at Cambridge where Francis Crick was working at the time [see The Storyof DNA: Part 1]. Your Majesties, Your Royal Highnesses, Ladies and Gentlemen.
When, in the early nineteenth century, Dalton had produced experimental proofs that matter consists of atoms it was not long before an explanation was sought of the forces that bind the atoms together. Berzelius was of the opinion that this chemical bond was caused by electrostatic attraction between the atoms; according to this belief, a bond was established between two atoms if one of the atoms was positively, and the other negatively charged. In 1819 when Berzelius presented his theory he could apply it almost exclusively to inorganic substances; only few organic substances were known as pure compounds, and the study of these was difficult due to their complicated and often insufficiently known composition. Berzelius, however, contrived to explain, with the help of the new theory, the bond conditions for a great number of inorganic substances, and could in this wav contribute in a high degree to a greater clarity in this field.
Even in inorganic chemistry, however, certain difficulties arose. How should one explain, for instance, how two hydrogen atoms unite to become a hydrogen molecule? In order to obtain attraction between atoms, one of the atoms must be positive and the other negative; but why should two atoms of the same kind possess charges with opposite sign? And when the knowledge of organic compounds increased, new difficulties arose. Berzelius, for example, found it necessary to assume that the hydrogen atom was always positive and the chlorine atom always negative. Now it was also found that in organic molecules a hydrogen atom could often be exchanged for a chlorine atom, which should be impossible if one was positive and the other negative.
With increased knowledge, problems that could not be explained by Berzelius' theory became more and more numerous, and the theory became discredited.
After the atomic theory had been accepted, it soon became apparent that another important object in the field of chemistry must be to determine not only the nature of the chemical bond but also how the atoms are arranged geometrically when they unite to form larger groups, such as molecules. Permit me to quote from a book, remarkable in its day, Die Chemie der Jetztzeit written in 1869 by the Swedish chemist Blomstrand:
"It is the important task of the chemist to imitate faithfully in his own way the elaborate constructions which we call chemical compounds, and in the erection of which the atoms have served as building stones, to determine as to number and relative position the points of attack at which one or the other of the atoms attaches itself to the other, in short, to determine the distribution in space of the atoms."
Blomstrand makes it the aim, therefore, to find the geometrical construction of substances, or what is nowadays called their structure.
At the end of the last century it became obvious that one had to consider several different kinds of chemical bond. Thus, the difficulties of the Berzelius theory were also explained. Berzelius' interpretation was in principle correct as regards a very important type of bond, but he had made the mistake of applying it also to bonds of a different type. After Bohr had introduced his atomic theory one could moreover with its help give a fairly satisfactory explanation of the Berzelius bond. As this bond occurs between electrically charged atoms, so-called ions, this bond type has often been called the ionic bond. The most typical ionic bonds unite the atoms in the crystals of simple salts.
The bond which above all others had prevented a general application of the Berzelius theory is now commonly known as the covalent bond. It occurs commonly when atoms unite to form a molecule and was once characterized by the famous American chemist Gilbert Newton Lewis as "the chemical bond". The bond between the two hydrogen atoms in a hydrogen molecule, which, as was said before, could not be explained by Berzelius' theory, is covalent.
For a long time it was difficult to explain the nature of the covalent bond. Lewis, however, succeeded in 1916 in showing that it is brought about by electrons - generally two - which are shared in common by two neighbouring atoms, thereby uniting them. Eleven years later Heitler and London were able to give a quantum-mechanical explanation of the phenomenon. An exact mathematical treatment of the covalent bond, however, was possible only in the simple case where only one electron unites the two atoms, and when these do not contain additional electrons outside the atomic nuclei. Even for the hydrogen molecule, which contains two electrons, the treatment cannot be absolutely exact, and in still more complicated cases the mathematical difficulties increase rapidly. It has, therefore, been necessary to use approximate methods, and the results depend to a large extent on the choice of suitable methods and the manner of their application.
Linus Pauling has actively contributed towards the development of these methods, and he has applied them with extreme skill. The results have been such as to be easily usable by chemists. Pauling has also eagerly sought to apply his views to a number of structures which have been experimentally determined during the last decades, both in his own laboratory in Pasadena and elsewhere. It is hardly necessary to mention that we have nowadays great possibilities of reaching Blomstrand's objective of determining the distribution of atoms in space. This is principally done by methods of X-ray crystallography involving an examination of how a crystal influences X-rays in certain respects, and then out of the effect seeking to determine how the atoms are placed in the crystal. Pauling's methods have been very successful and have led to observations which have further advanced the theoretical treatment.
But if the structure of a substance is too complicated it may become impossible to make a more direct determination of the structure with X-rays. In such cases it may be possible, from a knowledge of bond types, atomic distances and bond directions, to predict the structure and then examine whether the prediction is supported by the experiments. Pauling has tried this method in his studies of the structure of proteins with which he has been occupied during recent years. To make a direct determination of the structure of a protein by X-ray methods is out of the question for the present, owing to the enormous number of atoms in the molecule. A molecule of the coloured blood constituent hemoglobin, which is a protein, contains for example more than 8,000 atoms.
In the late nineteen thirties Pauling and his colleagues had already begun to determine with X-rays the structure of amino acids and dipeptides, that is to say, compounds of relatively simple structure containing what may be called fragments of proteins. From this were obtained valuable information - about atomic distances and bond directions. These values were supplemented by the determination of the probable limits of variation for distances and directions.
On this basis Pauling deduced some possible structures of the fundamental units in proteins, and the problem was then to examine whether these could explain the X-ray data obtained. It has thus become apparent that one of these structures, the so-called alpha-helix, probably exists in several proteins.
How far Pauling is right in detail still remains to be proved, but he has surely found an important principle in the structure of proteins. His method is sure to prove most productive in continued studies.
It is hardly necessary to question the practical use of the knowledge of the nature of chemical bonds and of the structure of substances. It is obvious that the properties of a substance must largely depend on the strength with which its atoms are united and the nature of the resulting structure. This I does not only apply to the physical properties of the substance, for instance hardness and melting point, but also to its chemical properties, that is to say how it participates in chemical reactions. If we know how certain atoms or groups of atoms are placed in a molecule we can often predict how the molecule should react under given conditions. And as every reaction results in the breaking of some bonds and the formation of others the result will largely depend on the relative strength of the different bonds.
Professor Pauling. Since you began your scientific career more than thirty years ago you have covered a diversity of subjects ranging over wide fields of chemistry, physics, and even medicine. It has been said of you that you have chosen to live "on the frontiers of science" and we chemists are keenly aware of the influence and the stimulative effect of your pioneer work.
Wide though your field of activity may be, you have devoted the greater part of your energy to the study of the nature of the chemical bond and the determination of the structure of molecules and crystals.
It is with great satisfaction, therefore, that the Royal Swedish Academy of Sciences has decided to award to you this year's Nobel Prize for Chemistry for your brilliant achievements in this fundamental field of chemistry.
On behalf of the Academy I wish to extend to you our heartiest congratulations, and now ask you to receive from the hands of His Majesty the King, the Nobel Prize for Chemistry for the year 1954.
Evolution is a well-confirmed process of biological change that produces diversity and coherent functionality by a variety of natural mechanisms.And here's what I posted in the comments on his blog.
The minimal definition of evolution is ..This is always an interesting discussion and the comments on Pharyngula make some good points. What do Sandwalk readers think of my definition?Evolution is a process that results in heritable changes in a population spread over many generations. [What Is Evolution?]The truly shocking thing is how many believers in evolution get it wrong. Unfortunately, that includes you PZ. Two of the absolutely essential features of any definition of evolution are: (1) it requires permanent genetic changes and (2) it is populations, not individuals, that evolve. Both of these restrictions are missing from your definition.
What this means is that there are lots of things covered by your definition that do not fit into the scientific definition of evolution. I'll leave it to your readers to come up with a list of such changes.
 The Centre for Inquiry Ontario is sponsoring a talk by Austin Dacey (photo below) on Friday night March 7th at the Beverley St. location [THE SECULAR CONSCIENCE: Why Belief Belongs in Public Life]. The talk starts at 7:30 pm and there's a reception for Friends of the Centre at 6pm.
The Centre for Inquiry Ontario is sponsoring a talk by Austin Dacey (photo below) on Friday night March 7th at the Beverley St. location [THE SECULAR CONSCIENCE: Why Belief Belongs in Public Life]. The talk starts at 7:30 pm and there's a reception for Friends of the Centre at 6pm.
Austin Dacey, Center for Inquiry's representative to the United Nations will speak in Toronto as one of the first stops on his book release tour.
Secularism has lost its soul. From Washington to the Vatican to Tehran, religion is a public matter as never before, and secular values-personal autonomy, toleration, separation of religion and state, and freedom of conscience-are attacked on all sides and defended by few. The godly claim a monopoly on the language of morality in public debate, while secular liberals stand accused of standing for nothing. Secular liberals have undone themselves. For generations, too many have insisted that questions of conscience-religion, ethics, and values-are "private matters" that have no place in public debate. Ironically, this ideology prevents them from subjecting religion to due scrutiny when it encroaches on individual rights, and from unabashedly advocating their own moral vision in politics for fear of "imposing" their beliefs on others.
In this incisive book, philosopher Austin Dacey calls for a bold rethinking of the nature of conscience and its role in public life. Inspired by an earlier liberal tradition he traces to Spinoza and John Stuart Mill, Dacey urges liberals to lift their self-imposed gag order and
defend a renewed secularism based on the objective moral value of conscience. He likens conscience to the free press in an open society: it is protected from coercion and control, not because it is private, but because it has a vital role in the public sphere. Conscience is free, but not liberated from shared standards of truth and right.
The Secular Conscience will be published by Prometheus Books in March 2008. In spring 2008, the author will bring his timely message directly to secularists, humanists, and skeptics. One of his first stops will be at the Centre for Inquiry Ontario in Toronto.
Austin Dacey, Ph.D., is a philosopher with the Center for Inquiry in New York City, where he serves as United Nations representative and a contributing editor at Skeptical Inquirer and Free Inquiry magazines. He teaches philosophy, ethics, and science education at Polytechnic University and State University of New York. He is the author of articles in numerous publications including the New York Times. His website is www.austindacey.com.
[Photo Credit: austindacey.com]
 Jonathan Wells (see photo) is one of the leading intelligent design creationists. (As we'll see, that says a lot about the intellectual vacuum that characterizes that cult.)
Jonathan Wells (see photo) is one of the leading intelligent design creationists. (As we'll see, that says a lot about the intellectual vacuum that characterizes that cult.) Third, Dardel and his colleagues made their discovery using protein crystallography. They were not guided by Darwinian evolutionary theory; in fact, they had no need of that hypothesis.When I first saw the Wells article I seriously wondered whether Jonathan Wells was mentally stable. It looks like he has become completely unhinged since the point of his article is so far from the truth that even a kindergarten student can recognize the lies. (Not surprisingly, the other intelligent design creationists were completely sucked into the lie.)
As principal investigator of the study under discussion, I’d like to strongly support the view advocated this page. In fact, I was completely amazed to see how our work has been misrepresented by M. Wells.Delicious. PZ Myers picked up on this and posted an article with the title Wells says something stupid again. Of course he did, that's why we call them IDiots.
Actually, we did indeed use darwinian evolution within this work (something unusual in structural biology). In order to obtain an enzyme with increased stability (a critical point for structural studies), we used selective pressure to obtain mutants of the enzyme. We selected for bateria with increased aminiglycoside resistance, by plating them on antibiotic containing medium. It turned out that some bacteria evolved such stabler enzymes variants which made this whole study possible !
Finally, I would not consider myself as a chemist, I got my PhD in molecular microbiology. It seems that M. Wells finds it easier to portray us as non-biologists, and hence implicitly as non-evolutionists.
As Johnny Cash reputedly once said, “It’s good to know who hates you, and it’s good to be hated by the right people.”You just can't make this stuff up. Wells is an IDiot. I intensely dislike Wells and the lying tactics he uses to promote his cult of intelligent design creationism. I hope that puts me among the "right people."
Darwinist bloggers P. Z. Myers and Ian Musgrave hate me. In fact, Myers writes, “My animus for Jonathan Wells knows no bounds.” Well, at least he (unlike Musgrave) spells my name right.
The most recent outbursts by Myers and Musgrave were provoked by my February 29 blog on Evolution News & Views, in which I predicted that Darwinists would try to take credit for a recent French discovery regarding antibiotic resistance. And indeed they did.
In the course of claiming credit for Darwinism, Musgrave claims that I completely misrepresent evolution, molecular biology, genetics and history. Wow. At least I get points for comprehensiveness. As proof of my misrepresentations, Musgrave cites Wikipedia, which everyone involved in this controversy knows is about as balanced and reliable on this issue as P.Z. Myers’s Pharyngula or The National Center for Science Miseducation’s Panda’s Thumb.
....
The principal researcher in the French study disagrees, and wrote to Musgrave’s blog that "we did indeed use Darwinian evolution within this work (something unusual in structural biology). In order to obtain an enzyme with increased stability (a critical point for structural studies), we used selective pressure to obtain mutants of the enzyme."
So the researchers used artificial selection to good advantage. But artificial selection is not Darwinism. People were using artificial selection for centuries before Darwin came along, and they didn’t need Darwin to explain it to them. Darwin argued that an analogous process also operates in natural populations – and so it does. But he and his devoted followers went much further and claimed that it also explains the origin of new species, organs and body plans, which it doesn’t.
[Photo Credit: Evolution News & Views]
Maurice, F., Broutin, I., Podglajen, I., Benas, P., Collatz, E. and Dardel, F. (2008) Enzyme structural plasticity and the emergence of broad-spectrum antibiotic resistance. EMBO Rep. 2008 Feb 22 [Epub ahead of print] [PubMed]
 The 24th edition of Mendel's Garden has just been posted on Bayblab [Mendel's Garden #24 - March 2008].
The 24th edition of Mendel's Garden has just been posted on Bayblab [Mendel's Garden #24 - March 2008].March has roared in like a lion; the freezing rain here may not be great for venturing outdoors, but it's perfect for a virtual walk through Mendel's Garden, your monthly carnival of genetics. Welcome to the 24th edition.
 SciBarCamp is starting in less than two weeks.
SciBarCamp is starting in less than two weeks. In the tradition of BarCamps, otherwise known as "unconferences", (see BarCamp.org for more information), the program is decided by the participants at the beginning of the meeting, in the opening reception. Presentations and discussion topics can be proposed here or on the opening night. SciBarCamp will require active participation; while not everybody will present or lead a discussion, everybody will be expected to contribute substantially - this will help make it a really creative event.Eva Amsen has suggested an interesting topic Ten Things Everyone Should Know About Science. Here's my quick list to get the discussion going ...

 This summer, a number of people, including scientists and philosophers, will gather at the Konrad Lorenz Institute in Altenberg, Austria to talk about evolution. There will be 16 of them and the theme of the meeting is to develop a new theory of evolution. You can read about it in an article by Susan Mazur on Scoop [Mazur: Altenberg! The Woodstock of Evolution?].
This summer, a number of people, including scientists and philosophers, will gather at the Konrad Lorenz Institute in Altenberg, Austria to talk about evolution. There will be 16 of them and the theme of the meeting is to develop a new theory of evolution. You can read about it in an article by Susan Mazur on Scoop [Mazur: Altenberg! The Woodstock of Evolution?]. 
A central issue in making a new theory of evolution is how large a role natural selection, which has come to mean the weeding out of traits that don't favor survival, gets to play.If the meeting was only about the role of chance and accident in evolution then it would be a valuable contribution to evolutionary theory. Instead, as the article makes clear, the "New Evolution" will probably focus on the opposite point of view. You can expect to see heavy emphasis on design, epigenetics, evo-devo and on self-organization as a fundamental principle. (I'm surprised Lynn Margulis wasn't invited.)
Natural selection was only part of Darwin's Origin of Species thinking. Yet through the years most biologists outside of evolutionary biology have mistakenly believed that evolution is natural selection.
A wave of scientists now questions natural selection's relevance, though few will publicly admit it. And with such a fundamental struggle underway, the hurling of slurs such as "looney Marxist hangover", "philosopher" (a scientist who can't get grants anymore), "crackpot", is hardly surprising.
When I asked esteemed Harvard evolutionary geneticist Richard Lewontin in a phone conversation what role natural selection plays in evolution, he said, "Natural selection occurs."
Lewontin thinks it's important to view the living world holistically. He says natural selection is not the only biological force operating on the composition of populations. And whatever the mechanism of passage of information from parent to offspring contributing to your formation, what natural selection addresses is "do you survive?"
 PZ Myers posted an article on the evolution of multicellularity [The choanoflagellate genome and metazoan evolution]. He begins with ...
PZ Myers posted an article on the evolution of multicellularity [The choanoflagellate genome and metazoan evolution]. He begins with ...What are the key innovations that led to the evolution of multicellularity, and what were their precursors in the single-celled microbial life that existed before the metazoa? We can hypothesize at least two distinct kinds of features that had to have preceded true multicellularity.PZ goes on to describe the genes in a single-cell eukaryote that diverged near the base of the animal phylum. The species is a choanoflagellate called Monosiga brevicollis.
The obvious feature is that cells must stick together; specific adhesion molecules must be present that link cells together, that aren't generically sticky and bind the organism to everything. So we need molecules that link cell to cell. Another feature of multicellular animals is that they secrete extracellular matrix, a feltwork of molecules outside the cells to which they can also adhere.
A feature that distinguishes true multicellular animals from colonial organisms is division of labor — cells within the organism specialize and follow different functional roles. This requires cell signaling, in which information beyond simple stickiness is communicated to cells, and signal transduction mechanisms which translate the signals into different patterns of gene activity.
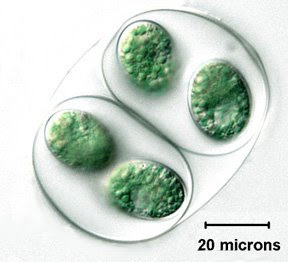 It's important to note that this single-cell organism and its multicellular animal relatives form a distinct clade that is separated from the fungi and plants. Since there are multicellular fungi and multicellular plants, the evolution of multicellularity must have occurred many times.
It's important to note that this single-cell organism and its multicellular animal relatives form a distinct clade that is separated from the fungi and plants. Since there are multicellular fungi and multicellular plants, the evolution of multicellularity must have occurred many times.  We can agree with his statement that two requirements of multicellularity are the ability of cells to stick together and the division of labor where cells differentiate to carry out specialized functions. Lest anyone imagines that these properties were invented by animals—or even by eukaryotes—let's look at some simple multicellular bacteria.
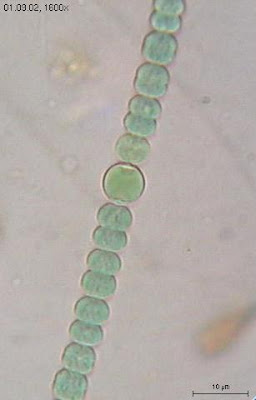
We can agree with his statement that two requirements of multicellularity are the ability of cells to stick together and the division of labor where cells differentiate to carry out specialized functions. Lest anyone imagines that these properties were invented by animals—or even by eukaryotes—let's look at some simple multicellular bacteria.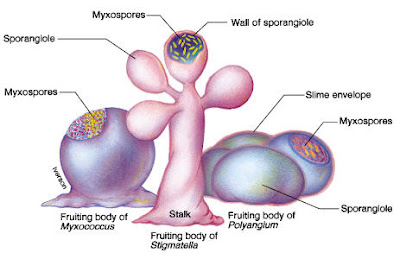 The myxobacteria are dramatic example of multicellular bacteria. That's Chondromyces crocatus shown in the photograph above left.
The myxobacteria are dramatic example of multicellular bacteria. That's Chondromyces crocatus shown in the photograph above left. [Photo Credits: Anabaena sphaerica from Wikipedia: Glaucocystis from Cyanobacteria slides: Chondromyces crocatus from The Myxobacteria Web page]
 Today's molecule is a cartoon depicting a particular conformation of molecules. Your task is to identify the structure. Be as specific as possible.
Today's molecule is a cartoon depicting a particular conformation of molecules. Your task is to identify the structure. Be as specific as possible. Jane Harris Zsovan posts on a blog called Design of Life. I don't read her blog on a regular basis but Denyse O'Leary recently linked to a post on how speciation doesn't agree with evolutionary theory [Hybridization One Key to Survival].
Jane Harris Zsovan posts on a blog called Design of Life. I don't read her blog on a regular basis but Denyse O'Leary recently linked to a post on how speciation doesn't agree with evolutionary theory [Hybridization One Key to Survival].Hybrids With Genetic Advantages A Problem for Darwinian TheoryThere are many different ways for two isolated populations to evolve into separate species. In many (most?) cases the two lineages diverge by random genetic drift and not just natural selection. The exact mutations leading to genetic incompatibility are most likely to arise by accident and become fixed in one of the lineages by drift.
Darwin's theory of natural selection requires offspring to diverge from a common ancestor to create new species. It requires genetic differences to increase as descendants adapt to their environmental niches.
It is this 'natural selection' and 'adaptation' that creates species. And, as the newly created species continue to adapt, they should become more different over time. Following this line of thought, hybrids should be less viable than their parents.
Sciencebase is this week proud to play host to the Gene Genie Blog Carnival thanks to an offer from Bertalan “Berci” Meskó over on the excellent ScienceRoll. For those who don’t already know, a Blog Carnival doesn’t usually involve a lot of be-costumed revellers dancing through the streets to the sound of the samba band, but is a gathering of like-minded bloggers brought together through the power of the tubular Interwebs to share their latest posts on a given subject.The beautiful logo was created by Ricardo at My Biotech Life.
 A column in yesterday's Toronto Star criticized the position of the so-called "New Atheists" [Erudite critic takes on new atheists]. The column was written by Stephen Scharper who frequently writes columns about religion. Scharper was formerly at St. Michael's College (Roman Catholic) in the University of Toronto where he was a Professor in the Department and Centre for the Study of Religion. He is currently a Professor in the Dept. of Anthropology at the University of Toronto Mississauga Campus but he maintains a cross-appointment to the Department and Centre for the Study of Religion.
A column in yesterday's Toronto Star criticized the position of the so-called "New Atheists" [Erudite critic takes on new atheists]. The column was written by Stephen Scharper who frequently writes columns about religion. Scharper was formerly at St. Michael's College (Roman Catholic) in the University of Toronto where he was a Professor in the Department and Centre for the Study of Religion. He is currently a Professor in the Dept. of Anthropology at the University of Toronto Mississauga Campus but he maintains a cross-appointment to the Department and Centre for the Study of Religion. A couple of years ago, I was on a televised panel with a man who claimed that religions were to blame for most of the death and destruction throughout history, and that, by extension, religious people were more inherently violent than secular folks.I don't agree with the extreme view pictured by Stephen Scharper and neither do most atheists. It is simply not true that religious people are inherently more violent than atheists. On the other hand, it is simply not true that believers are more moral than non-believers.
The implication of his claim was that discrimination against religious adherents would not only be acceptable, but advisable in such matters as job hiring, policy formation, and policing.
Prejudice against people of faith was subtly being proclaimed as an inherent good.
That panelist's view, extreme as it appears, may have some powerful mainstream resonance in a spate of recent publications from the so-called "new atheists."
 Scharper goes on to praise a recent book by John Haught (God and the New Atheism: A Critical Response To Dawkins, Harris And Hitchens). I haven't read this book but from what Scharper says it sounds like the same-old, same-old that we've heard dozens of time before [e.g. Alister McGrath's Defense of Religion, Propaganda Techniques: Shift the Burden of Proof, Alister McGrath].
Scharper goes on to praise a recent book by John Haught (God and the New Atheism: A Critical Response To Dawkins, Harris And Hitchens). I haven't read this book but from what Scharper says it sounds like the same-old, same-old that we've heard dozens of time before [e.g. Alister McGrath's Defense of Religion, Propaganda Techniques: Shift the Burden of Proof, Alister McGrath].Haught, a senior fellow at the Woodstock Theological Center at Georgetown University, adroitly distills the common themes from this troika [Dawkins, Harris, Hitchens], which suggests that outside of nature, "there is no God, no soul, and no life beyond death," that the universe has no overarching purpose, that all life developments can be explained by science, and that faith in God is the source of countless evils and should thus be jettisoned on moral grounds.Now, let's be absolutely clear about this argument. It is completely bogus. This is not a debate about religion, it's a debate about the existence of supernatural beings. Atheists are simply not interested in debating how many angels can dance on the head of a pin. They want to debate the evidence for angels. Atheists don't want to debate why God and evil can coexist. They want to debate whether God exists at all. We don't care about the extensive literature on the interpretation of Chapters 1 & 2 of Genesis. It's irrelevant.
What Haught finds ironic is that the new atheists have little interest in atheism at all, and don't engage in the main philosophical debates raised by atheistic giants such as Karl Marx, Friedrich Nietzsche, Jean-Paul Sartre and Albert Camus. Instead, they base their notion of religion on the creationism of a select band of fundamentalists. In short, they adopt a theologically bereft caricature of religion.
I have considered the impudent accusations of Mr Dawkins with exasperation at his lack of serious scholarship. He has apparently not read the detailed discourses of Count Roderigo of Seville on the exquisite and exotic leathers of the Emperor's boots, nor does he give a moment's consideration to Bellini's masterwork, On the Luminescence of the Emperor's Feathered Hat. We have entire schools dedicated to writing learned treatises on the beauty of the Emperor's raiment, and every major newspaper runs a section dedicated to imperial fashion; Dawkins cavalierly dismisses them all. He even laughs at the highly popular and most persuasive arguments of his fellow countryman, Lord D. T. Mawkscribbler, who famously pointed out that the Emperor would not wear common cotton, nor uncomfortable polyester, but must, I say must, wear undergarments of the finest silk.Here's my challenge to Stephen Scharper: if you know of a sophisticated argument for the existence of God that hasn't already been addressed, and refuted, by atheists, then here's your chance to present it. I notice that you didn't mention it in your column and none of your colleagues have actually presented one of those secret theological debating points that you claim exist in the corridors of seminaries and departments of philosophy or religion.
Dawkins arrogantly ignores all these deep philosophical ponderings to crudely accuse the Emperor of nudity.
Personally, I suspect that perhaps the Emperor might not be fully clothed — how else to explain the apparent sloth of the staff at the palace laundry — but, well, everyone else does seem to go on about his clothes, and this Dawkins fellow is such a rude upstart who lacks the wit of my elegant circumlocutions, that, while unable to deal with the substance of his accusations, I should at least chide him for his very bad form.
Until Dawkins has trained in the shops of Paris and Milan, until he has learned to tell the difference between a ruffled flounce and a puffy pantaloon, we should all pretend he has not spoken out against the Emperor's taste. His training in biology may give him the ability to recognize dangling genitalia when he sees it, but it has not taught him the proper appreciation of Imaginary Fabrics.
[Photo Credit: The photograph of Professor Stephen Scharper is from the University of Toronto News]
 My daughter Jane and her husband Michael live in Brussels. Last weekend we visited them and they took us on a walking tour of downtown Brussels.
My daughter Jane and her husband Michael live in Brussels. Last weekend we visited them and they took us on a walking tour of downtown Brussels.  The very first stop was a place that sold Belgian waffles. There are dozens of these shops with windows open to the street. It's the Brussels equivalent of Tim Horton's. The waffles were delicious and I would have eaten several except that I was saving up for even better things later on.
The very first stop was a place that sold Belgian waffles. There are dozens of these shops with windows open to the street. It's the Brussels equivalent of Tim Horton's. The waffles were delicious and I would have eaten several except that I was saving up for even better things later on. Eventually we reached a delightful part of the city with narrow medieval streets filled with restaurants. Our goal was a fascinating restaurant called Chez Leon. The specialty is mussels and frites (French fries), a meal that can't be found anywhere else in the world as far as I know.
Eventually we reached a delightful part of the city with narrow medieval streets filled with restaurants. Our goal was a fascinating restaurant called Chez Leon. The specialty is mussels and frites (French fries), a meal that can't be found anywhere else in the world as far as I know.  Some of you may be curious about the price of such a meal. The mussels and frites were 22.50 € and that works out to about $33 in Canadian or American currency. This seems expensive but it's not out of line with many other prices in the local restaurants. I really don't know how the French and Belgians can afford to eat out so much. Their salaries don't seem to be higher than what we are used to in North America. I guess they just have different priorities.
Some of you may be curious about the price of such a meal. The mussels and frites were 22.50 € and that works out to about $33 in Canadian or American currency. This seems expensive but it's not out of line with many other prices in the local restaurants. I really don't know how the French and Belgians can afford to eat out so much. Their salaries don't seem to be higher than what we are used to in North America. I guess they just have different priorities.  You can't walk very far in Brussels without encountering a chocolate shop. (We visited several.) Everyone has their favorite but I'm told that Neuhaus chocolate is widely believed to be the best Belgian chocolate. The Belgians are very proud of their chocolate. They think it's superior to Swiss chocolate. I'm not going to take sides on that one since I lived in Switzerland for many years and still have many friends there.
You can't walk very far in Brussels without encountering a chocolate shop. (We visited several.) Everyone has their favorite but I'm told that Neuhaus chocolate is widely believed to be the best Belgian chocolate. The Belgians are very proud of their chocolate. They think it's superior to Swiss chocolate. I'm not going to take sides on that one since I lived in Switzerland for many years and still have many friends there. I'm used to seeing chocolate shops but the really surprising shops were the ones that sell beer. We saw a dozen stores that specialized in beers with a huge emphasis on Belgian beers. These stores look like wine stores except that it's bottles of beer that are lined up on the shelves.
I'm used to seeing chocolate shops but the really surprising shops were the ones that sell beer. We saw a dozen stores that specialized in beers with a huge emphasis on Belgian beers. These stores look like wine stores except that it's bottles of beer that are lined up on the shelves.  After a long day we stopped at one of the Häagen-Dazs Cafés for chocolate fondue. We were served a plate full of small balls of ice cream of various flavors. These can be dipped in a creamy pot of rich chocolate. The ice cream was accompanied by a plate of fresh fruit and pieces of muffin that also went well with the hot chocolate. All in all it was an excellent way to end our delicious tour of Brussels.
After a long day we stopped at one of the Häagen-Dazs Cafés for chocolate fondue. We were served a plate full of small balls of ice cream of various flavors. These can be dipped in a creamy pot of rich chocolate. The ice cream was accompanied by a plate of fresh fruit and pieces of muffin that also went well with the hot chocolate. All in all it was an excellent way to end our delicious tour of Brussels.