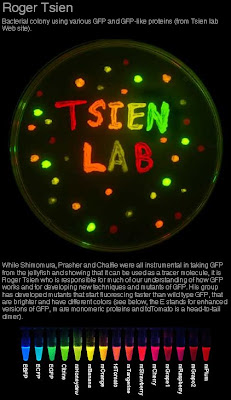
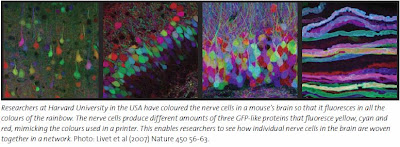
The Nobel Prize in Physiology or Medicine 1966
"for their discoveries concerning the specificity of the cell mediated immune defence"
 Peter C. Doherty (1949 - ) and Rolf M. Zinkernagel (1940 - ) won the Noble Prize in 1996 for their work on the mechanism of cellular immunity (cell-mediated immunity). They were the ones who figured out how T-cells can recognize and kill cells that are infected with a virus.
Peter C. Doherty (1949 - ) and Rolf M. Zinkernagel (1940 - ) won the Noble Prize in 1996 for their work on the mechanism of cellular immunity (cell-mediated immunity). They were the ones who figured out how T-cells can recognize and kill cells that are infected with a virus. This is part of the modern era of Nobel Prize awards where the Nobel Foundation is doing a wonderful job of explaining the awards to a scientifically literate general public. Here's the 1996 Press ReleaseTHEME:
Nobel Laureates
Summary
Peter Doherty and Rolf Zinkernagel have been awarded this year's Nobel Prize in Physiology or Medicine for the discovery of how the immune system recognizes virus infected cells. Their discovery has, in its turn, laid a foundation for an understanding of general mechanisms used by the cellular immune system to recognize both foreign microorganisms and self molecules. This discovery is therefore highly relevant to clinical medicine. It relates both to efforts to strengthen the immune response against invading microorganisms and certain forms of cancer, and to efforts to diminish the effects of autoimmune reactions in inflammatory diseases, such as rheumatic conditions, multiple sclerosis and diabetes.
The two Nobel Laureates carried out the research for which they have now been awarded the Prize in 1973-75 at the John Curtin School of Medical Research in Canberra, Australia, where Peter Doherty already held his position and to which Rolf Zinkernagel came from Switzerland as a research fellow. During their studies of the response of mice to viruses, they found that white blood cells (lymphocytes) must recognize both the virus and certain self molecules - the so-called major histocompatibility antigens - in order to kill the virus-infected cells. This principle of simultaneous recognition of both self and foreign molecules has since then constituted a foundation for the further understanding of the specificity of the cellular immune system.
The background to the Laureates' research
The immune system consists of different kinds of white blood cells, including T- and B- lymphocytes whose common function is to protect the individual against infections by means of eliminating invading microorganisms and infected cells. At the same time they must avoid damaging the own organism. What is required is a well developed recognition system that enables lymphocytes to distinguish between on the one hand microorganisms and infected cells, and on the other, the individual´s normal cells. In addition, the recognition system must be able to determine when white blood cells with a capacity to kill should be activated.
In the early 1970s when Peter Doherty and Rolf Zinkernagel had begun their scientific work within immunology, it was possible to distinguish between antibody-mediated and cell- mediated immunity. It was known that antibodies that are produced by B-lymphocytes are able to recognize and eliminate certain microorganisms, particularly bacteria. Far less was known about recognition mechanisms in the cellular immune system, for instance in conjunction with the killing of virus-infected cells by T-lymphocytes. One area where cellular immunity had previously been studied in some detail was, however, transplantation biology. It was known that T-lymphocytes could kill cells from a foreign individual after recognition of certain molecules - the major histocompatibility antigens - in the transplant.The discovery
Rolf Zinkernagel and Peter Doherty used mice to study how the immune system, and particularly T -lymphocytes, could protect animals against infection from a virus able to cause meningitis. Infected mice developed killer T-lymphocytes, which in a test-tube could kill virus- infected cells. But there was an unexpected discovery: the T-lymphocytes, even though they were reactive against that very virus, were not able to kill virus-infected cells from another strain of mice. What decided whether or not a cell was eliminated by these killer lymphocytes was not only if they were infected with the virus, but also if they carried the "correct" variant of histocompatibility antigens, those of the infected mouse itself. Zinkernagel's and Doherty's findings, which were published in Nature in 1974 (1,2), demonstrated conclusively the requirement for the cellular immune system to recognize simultaneously both 'foreign' molecules (in the present case from a virus) and self molecules (major histocompatibility antigens). What also became obvious was the important function of the major histocompatibility antigens (in man called HLA-antigens) in the individual´s normal immune response and not only in conjunction with transplantation.
The discovery has given an impetus to later research
Zinkernagel's and Doherty´s findings had an immediate impact on immunological research. The wide relevance of their observations concerning the specificity of the T-lymphocytes became apparent in many contexts, both in regard to the ability of the immune system to recognize microorganisms other than viruses, and in regard to the ability of the immune system to react against certain kinds of self tissue. To explain their findings, the two scientists subsequently devised two models; one model was based on a single recognition of 'altered self''(when the histocompatibility antigen has been modified through association with a virus), the other on a 'dual recognition' of both foreign and self. (Fig.) Both the experimental findings and the theoretical models became immensely important in later research. Within a few years, it had been demonstrated that the set of the T- lymphocytes that are allowed to mature and survive in an individual is determined by the ability of the cell to recognize the transplantation antigens of the individual. Therefore, the principle of simultaneous recognition is essential for the ability of the immune system to distinguish between 'self' and 'non-self'.
Further molecular research has both confirmed Zinkernagel's and Doherty's models and clarified the structural basis of their discovery - that a small part (a peptide), for example from a virus, is directly bound to a defined variable part of the body´s own histocompatibility antigens, and that this complex is what is recognized by the specific recognition molecules of T- lymphocytes (T-cell receptors). Taken in all, the clarification of the recognition mechanisms of the T-cells within the cellular immune system has fundamentally changed our understanding of the development and normal function of the immune system and, in addition, has also provided new possibilities for the selective modification of immune reactions both to microorganisms, and to self tissues.Figure legend: The figure describes how a killer T lymphocyte must recognize both the virus antigen and the self histocompatibility antigen molecule in order to kill a virus-infected target cell. The figure is a modification of the figure published by Zinkernagel and Doherty already 1974 (in Nature 251, p 547).
Relevance for clinical medicine
Many common and severe diseases depend on the function of the cellular immune system and consequently on its mechanisms for specific recognition. Although this naturally applies to infectious diseases, this is also true of a number of chronic inflammatory conditions such as rheumatic diseases, diabetes and multiple sclerosis. Where infectious diseases are concerned, the new knowledge provides a better platform for the construction of new vaccines; one can ascertain exactly what parts of a microorganism are recognized by the cellular immune system, and can specifically focus the production of the vaccine on those parts. Furthermore, regard is paid to the fundamental principles formulated by Doherty and Zinkernagel in trials with vaccination against the emergence of metastases in certain forms of cancer. In many chronic inflammatory diseases, better explanations have been provided for the associations between disease susceptibility and the histocompatibility antigen type carried by an individual. The research that followed from the now awarded discovery has also provided openings for selectively diminishing or altering immune reactions that play a central role in inflammatory diseases.
References
1. Zinkernagel RM, Doherty PC. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngenic and semiallogeneic system. Nature 248, 701- 702, 1974.
2. Zinkernagel RM, Doherty PC. Immunological surveillance against altered self components by sensitised T lymphocytes in lymphocytic choriomeningitis. Nature 251, 547-548, 1974.
3. Doherty PC, Zinkernagel RM. A biological role for the major histocompatibility antigens. Lancet, 1406-1409, 1975.
4. Zinkernagel RM, Doherty PC. MHC restricted cytotoxic T cells: Studies on the biological role of polymorphic major transplantation antigens determining T cell restriction specificity. Advances in Immunology 27, 51-177, 1979.
The images of the Nobel Prize medals are registered trademarks of the Nobel Foundation (© The Nobel Foundation). They are used here, with permission, for educational purposes only.