It's the acid that does it and that acid is caused by synthesis of lactic acid taking place during anaerobic exercise, or so the story goes. That happens under extreme conditions when the energy needed by working muscles exceeds the ability to produce it by normal aerobic oxidation. It all sounds so logical ... and so biochemical.
It's all a myth. Lactic acid has nothing to do with acidosis (the buildup of acid in the muscles). In fact, it's not even clear that acidosis is the problem, but let's deal with that another time.
Assuming that acid buildup in muscles is what causes the pain of the long distance runner, where does that acid comes from? In order to answer that question we need a brief lesson on acids.
Acids are molecules that can give up a hydrogen ion (H+), or proton. Hydrochloric acid (HCl) and acetic acid are classic examples. They can both dissociate into H+ and a negatively charged ion; either a chloride ion in the case of hydrochloric acid (Cl-) or an acetate ion (CH3COO-) in the case of acetic acid.
The strength of an acid depends on how easily it dissociates into hydrogen ions. Hydrochloric acid is a strong acid because it dissociates almost completely and acetic acid is a weak acid because it only partially dissociates in water.
The concentration of hydrogen ions is what makes solutions acidic and we describe that concentration by referring to the pH of the solution where the "H" stands for hydrogen ions. The pH scale is a log scale and it is the negative log of the hydrogen ion concentration (don't ask). Solutions with low pH have very high concentrations of hydrogen ions.
Muscles need biochemical energy to do their work and that energy is supplied by ATP, the common energy currency in the cell. As ATP is used up, it needs to be regenerated and the quickest way to do that is to makes make more ATP using creatine phosphate, a high energy molecule stored in muscle cells. When the creatine phosphate is depleted, muscle cells mobilize their store of glycogen converting it to glucose that is then metabolized by the glycolysis pathway. The end products of this pathway are pyruvate, ATP, and NADH. The ATP produced during glycolysis is used by the muscle cells.
Under normal conditions, pyruvate enters a pathway called the citric acid cycle and this pathway regenerates NAD+ from NADH so that glycolysis can continue. This reaction is coupled to synthesis of more ATP by mitochondria, a process that requires oxygen.
Here's where things get tricky for athletes. Their muscles are often working so hard that the resupply of ATP by glycolysis and the citric acid cycle can't keep up with the oxygen supply, no matter how hard the athletes are breathing. Pyruvate begins to accumulate in the muscle cells because it can't be metabolized quickly enough in the citric acid cycle.
Under these conditions, pyruvate is converted to lactate in order to generate more NAD+ so that glycolysis can continue. This is often referred to as anaerobic metabolism. Look closely at the reaction.
The product of this reaction is lactate, not lactic acid. Lactate is not an acid because it can't give up a proton. The overall reaction doesn't produce acid (H+), it actually consumes it. Muscle cells do not accumulate lactic acid, they accumulate lactate and that's not the same thing.
So what causes acidosis in muscles? It may come, in part, from the reaction that consumes ATP but this can't be the whole story because ATP is rapidly regenerated, using up the hydrogen ion.
Some of the acidity may be indirectly due to a buildup of lactate affecting buffering capacity but this doesn't seem to be a likely cause of acidosis.
The important point is that lactic acid is not produced in muscles so it can't be the source of acidosis. This has been known in the scientific literature for twenty years but it doesn't seem to have entered the biochemistry textbooks until recently. The myth of lactic acid has been debunked in newspapers and science magazines but it's still believed by athletic coaches and trainers and by the athletes themselves. It doesn't really matter since training is able to overcome the limits of muscle metabolism whatever the cause. It's likely that training and hard exercise increase the number of mitochondria in muscle cells and this could be the real benefit since it allows for more aerobic metabolism.
Here's just a small sample of articles debunking the lactic acid myth. Let me know if you find more.
Lactate: Not Guilty as Charged
Lactic Acid
Lactic Acid Is Not Muscles' Foe, It's Fuel
[Encouragement Credit: Ms. Sandwalk made me post this. She got tired of hearing me yell at the television and she was afraid I'd throw my textbook at the screen.]
[Image Credits: Moran, L.A., Horton, H.R., Scrimgeour, K.G., and Perry, M.D. (2012) Principles of Biochemistry 5th ed., Pearson Education Inc. (various pages) [Pearson: Principles of Biochemistry 5/E] ©2012 Pearson Education Inc.]

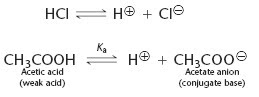


33 comments :
Thats interesting as it debunks a commonly held belief.
It required, you said its been twenty years, a closer investigation of the data and gaining more data.
As a YEC creationist I say this principal can be applied to evolutionary biology and geology.
Closer investigation of data and new data can overthrow wrong, though plausibly sounding, ideas.
YEC and ID both have been doing this a great deal in the last twentu years and believe wrong ideas wil be shown wrong.
takes a while to reach large audiences.
Yec and id have no evidence and which god ? Zeus ? Thor ?
I'm sure the YEC and ID crowd will overthrow evolutionary ideas as soon as they find their dictionaries and learn to use spell check.
Oh, wait, they won't overthrow evolutionary ideas because they don't do any original research, contra Robert Byers' assertion. Some evolutionary ideas will lose favor as scientific evidence accumulates against them while other evolutionary ideas gain more scientific evidence favoring them, but none of it will likely favor YEC or ID. Evolution happened and is happening. There is no evidence for YEC or ID. Deal with it.
"The pH scale is a log scale and it is the negative log of the hydrogen ion concentration (don't ask)."
But inquiring minds want to know...
Fascinating from the biochemistry point of view.
Less so from the athlete's! All we care about is that running over the aerobic threshold causes burn (which I suppose is why we like the "acid" part of the lactic acid myth, it's a good mental image"
That last lap of a 1500 when when everything is locking up... telling myself it's lactate, not lactic acid doesn't help! Telling myself I should have trained harder does!
No seriously, nice blog, I'm grateful to Planet Atheism for putting your new posts in front of me
"YEC and ID both have been doing this a great deal"
HAHAHAHAHAHAHA.
Oh...
Has the putative decrease in pH ever been measured in an athlete following a sprint, or say a running back after a play? Also, could the increase in CO2 account for a sufficient decrease in pH over the short time of a 100m sprint?
Also, at least one of my physiology texts noted that lactate build-up cannot be responsible for "the burn" and that the burn is more likely due to micro-tears in muscle cell membranes and connective tissue. I'm not sure on what time scale over which the molecular signals from damaged tissue would operate to trigger pain, if it is short enough to produce the burn that comes with fatigue.
The muscle pain that lasts for days is a separate problem. Back in the olden days (1990s) there were exercise physiologists who attributed this to lactic acid buildup but biochemists knew even then that any lactate accumulating in muscle was quickly metabolized or exported to the bloodstream [The Cori Cycle].
That myth was debunked ten years ago although you'll still find it on the internet. (Believe it or not, there is false information on the internet!)
But inquiring minds want to know...
I know a good book you could read. :-)
Seriously, do you think there's enough interest? Is anyone really curious about the little "p" in pH or the reason why 7.0 is neutral and not 19.2 or 1.8?
Doesn't adding lactate shift the equilibrium of latic acid dissociation and either decrease equilibrium the pH of the lactate buffer system or increase the concentration of lactic acid by LaChâtelier's Principle?
In an isolated system if you add the conjugate base of a weak acid (e.g. lactate) that should reduce the concentration of free hydrogen ions and increase the pH.
Cells are not isolated systems. They are full of anionic molecules like lactate and pyruvate. All the intermediates in glycolysis and the citric acid cycle, for example, are like lactate and so are all the amino acids. The transformation of one conjugate base, like pyruvate, into another, like lactate, isn't going to change the pH.
"[Encouragement Credit: Ms. Sandwalk made me post this. She got tired of hearing me yell at the television and she was afraid I'd throw my textbook at the screen.]"
I think you are preaching to the choir here on your blog. I was actually "encouraging" you to inform the Athletic Doctor employed by CTV of his misinformation. He's still yapping, so you had better use your new-found ability to tweet him :) You won the debate on Dr. Spock (the baby guru) years ago, I recanted, so now you need to revolutionize the athletic world. Good luck.
Is acetly CoA charged? Does it have appreciable acid-base chemistry in aqueous solution?
The important thing to understand about metabolism and pathways is the notion of steady-state. The concentrations of metabolites in the common pathways do not change. No matter what the conditions of the muscle cell, the concentration of pyruvate, acetyl-CoA, and all the other intermediates in glycoysis and the citric acid cycle remain relatively constant [Better Biochemistry: Near-Equilibrium Reactions].
It doesn't really matter if acetyl-CoA is charged or not.
Just on its own, yes. That would be a fascinating post.
Hey YEC creationist, it would be better if you could first get your language right - don't write principal (that was the guy who spanked you in school), when you mean principle. Not that it is going to seriously help you prove the earth is 5000 or 500 or 50 years old...
Okay, that makes two of you. It's all the encouragement I need since it represents a substantial percentage of my readership.
:-)
"YEC and ID both have been doing this"
Doing what, exactly? Synthesizing plausible sounding, wrong ideas?
What are the alternative hypotheses to acidosis for exertion-caused muscle pain?
"Muscle cells do not accumulate lactic acid, they accumulate lactate and that's not the same thing."
True.
"lactic acid is not produced in muscles so it can't be the source of acidosis."
False.
Muscles are organs, not cells. Lactic acidosis is empirical. As I understand it, lactate is exported from muscle cells by a cotransporter that also excretes a proton (to maintain intracellular electrical neutrality). Therefore muscle tissue does indeed accumulate lactic acid, but extracellularly.
This does not mean that it has anything to do with fatigue or 'feeling a burn'.
We agree that muscle cells do not accumulate lactic acid.
Most of the lactate formed during intense activity is reconverted to pyruvate and then to glycogen after the period of intense activity is over.
It's true that a considerable amount of lactate is exported to the blood stream where it's taken up by liver cells and transformed into glycogen.
Are you saying that the export of lactate from muscle cells is accompanied by export of a proton but the uptake of lactate by liver cells does not require the uptake of a proton and this makes the blood acidic? Where do all the protons in the muscle cytoplasm come from?
Interesting comparative angle - acidosis after exertion is a much bigger problem for ectotherms, particularly large ones, to the point that one of the big issues in capturing large crocodiles (apart from the whole "don't get eaten" thing) is that, if they struggle too long, they can develop metabolic acidosis strong enough to kill them.
It's also been suggested that the extensive dermal bone of modern crocs and early tetrapods functions as a buffer against metabolic acidosis.
Not really my field of specialization (I teach general physiology), but my understanding is that essentially all transport of lactate across cell membranes, on the way in or out, is accompanied by proton cotransport. So no, the protons enter the hepatocytes with the lactate.
link
link
As for the source of muscle-cell protons, hell I don't know; you're the biochemist! My guess would be carbonic anhydrase but I don't really know.
Yes, great point.
It's further been suggested that the vertebrates's use of calcium phosphate salts in bone instead of calcium carbonate is attributable to the earlier evolution of anaerobic support of exercise with lactate fermentation. Acidity is not kind to calcium carbonate.
NADH is a weak acid. isn't it ? i can see that in the reaction it loses the H+ and it is supposed to be NAD- in the products. but it is written as NAD+ . hope you can explain it.
The reaction is an oxidation-reduction reaction. Two electrons are transferred from NADH to lactate in the form of a hydride ion (H plus two electrons). You can read all about it on Wikipedia: Nicotinamide adenine dinucleotide.
I've realised that lactate acidosis couldn't be explanatory especially after realising that pyruvate has an even lower pKa, meaning that conversion of pyruvate to lactate should actually buffer some acid.
However, glycolysis starts out with 6 hydroxyl-functions ready to be oxidized, and pyruvate winds up with a carboxyl function that's dissociated. The H+ in that case is generated by the 5. step of glycolysis, or the phosphoglycerine-aldehydedehydrogenase reaction. Also, respiratory chain produces CO2, which diffuses out of the cell and shifts the ratio of CO2/HCO3- + H+, releasing more protons.
Well, there's your acidosis, which happens in pretty much any metabolically active cell, including tumors.
Hi!
I'm getting my masters in Biochemistry at Georgetown and planning to continue with a PhD in biochemistry and physiology. I just wanted to thank you for writing this article! This is what inspires me to learn and get excited about biochemistry. I love to see the link of biochemistry back to the physical experience. Thank you so much!
Shay
Very detail information provided on biochemistry. The conclusion which I got is not the lactic acid but the excess production of H+ Ions which cease the metabolic reactions in cells and can cause soreness. Atheletes should maintain the quantity of lactate produce in the body. More information which I got in layman language is at the article on lactic acid at Runnersworld Lactic Acid. You can also get some related articles on prevention of lactate.
Yes! Students ask little questions like this all the time. Where does the p come from? How did all of the ic and ous and ate and ite endings get developed for ions and acids.
Should we infer from this article that the source of muscle burn after exercise is from carbonic acid / CO2 that is the byproduct of aerobic exercise, rather than the lactate that is the output of anaerobic exercise?
Hey Larry! I'm a new reader and I love your blog; I have been binge reading several of your posts and went down a photosynthesis wormhole after reading your point that photosynthesis makes ATP and NADPH but not necessarily carbohydrates 🤯. I'm a high school biology teacher, and I'm wondering, have you ever heard of George Brooks? He says that lactate itself is the end product of glycolysis and that it (lactate) is produced all the time in our body in fully aerobic conditions. I'd love your take on this! https://pmc.ncbi.nlm.nih.gov/articles/PMC9124087/
https://www.sciencedirect.com/science/article/pii/S2095254620300193
Hey Larry! Sorry if you're seeing this post twice; I'm not sure if my first post went through. 'm wondering if you have ever heard of George Brooks and his lactate shuttle theory? He thinks lactate is the end product of glycolysis, not pyruvate, and that lactate is produced under fully aerobic conditions. I'd LOVE for you to dig into this and let us know your thoughts!!! Julia
Julia: Comments on older posts have to be approved by me. Sorry it took so long.
I'm not very interested in human biochemistry and physiology and I'm not a big fan of generalizations based on what happens in humans.
However, I see nothing wrong with the idea that some lactate is produced frequently in some human tissues. The idea that lactate and not pyruvate is the main product of glycolysis is silly.
Post a Comment